Loss of CD103+ intestinal dendritic cells during colonic inflammation
- PMID: 20039445
- PMCID: PMC2799913
- DOI: 10.3748/wjg.v16.i1.21
Loss of CD103+ intestinal dendritic cells during colonic inflammation
Abstract
Aim: To investigate possible differences in dendritic cells (DC) within intestinal tissue of mice before and after induction of colitis.
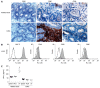
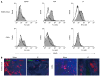
Methods: Mucosal DC derived from intestinal tissue, as well as from mesenteric lymph nodes and spleen, were analyzed by fluorescence activated cell sorting (FACS) analysis. Supernatants of these cells were analyzed for secretion of different pro- and anti-inflammatory cytokines. Immunohistochemistry and immunofluorescence were performed on cryosections of mucosal tissue derived from animals with colitis as well as from healthy mice.
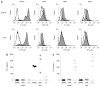
Results: It was shown that DC derived from healthy intestinal lamina propria (LP) represented an immature phenotype as characterized by low-level expression of costimulatory cytokines. In contrast to DC from spleen and mesenteric lymph nodes (MLN) that secreted proinflammatory cytokines, LP-DC produced high levels of the anti-inflammatory cytokine IL-10. After induction of murine colitis in a CD4(+)CD62L(+) transfer model or in chronic dextran sulfate sodium-colitis, a marked increase of activated CD80(+) DC could be observed within the inflamed colonic tissue. Interestingly, in contrast to splenic DC, a significant population of DC within MLN and colonic LP expressed the mucosal integrin CD103 which was lost during colitis.
Conclusion: The constitutive secretion of anti-inflammatory cytokines by immature DC within the intestinal LP might regulate the homeostatic balance between mucosal immunity and tolerance. CD103(+) DC could mediate this important function.
Figures
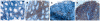
Similar articles
-
Enterocyte dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin expression in inflammatory bowel disease.World J Gastroenterol. 2015 Jan 7;21(1):187-95. doi: 10.3748/wjg.v21.i1.187. World J Gastroenterol. 2015. PMID: 25574091 Free PMC article.
-
Small intestine CD11c+ CD8+ T cells suppress CD4+ T cell-induced immune colitis.Am J Physiol Gastrointest Liver Physiol. 2011 Jun;300(6):G939-47. doi: 10.1152/ajpgi.00032.2010. Epub 2011 Mar 24. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21436315 Free PMC article.
-
Mesenteric lymph node CD11b- CD103+ PD-L1High dendritic cells highly induce regulatory T cells.Immunology. 2017 Sep;152(1):52-64. doi: 10.1111/imm.12747. Epub 2017 Jun 1. Immunology. 2017. PMID: 28423181 Free PMC article.
-
P-selectin glycoprotein ligand-1 modulates immune inflammatory responses in the enteric lamina propria.J Pathol. 2011 Jun;224(2):212-21. doi: 10.1002/path.2850. Epub 2011 Mar 22. J Pathol. 2011. PMID: 21432853
-
Intestinal CD103(+)CD11b(-) dendritic cells restrain colitis via IFN-γ-induced anti-inflammatory response in epithelial cells.Mucosal Immunol. 2016 Mar;9(2):336-51. doi: 10.1038/mi.2015.64. Epub 2015 Jul 15. Mucosal Immunol. 2016. PMID: 26174764 Free PMC article.
Cited by
-
Whole β-glucan particle attenuates AOM/DSS-induced colorectal tumorigenesis in mice via inhibition of intestinal inflammation.Front Pharmacol. 2023 Jan 12;14:1017475. doi: 10.3389/fphar.2023.1017475. eCollection 2023. Front Pharmacol. 2023. PMID: 36713833 Free PMC article.
-
Dual immune functions of IL-33 in inflammatory bowel disease.Histol Histopathol. 2020 Feb;35(2):137-146. doi: 10.14670/HH-18-149. Epub 2019 Jul 11. Histol Histopathol. 2020. PMID: 31294456 Review.
-
Interleukin-4 Inhibits Regulatory T Cell Differentiation through Regulating CD103+ Dendritic Cells.Front Immunol. 2017 Mar 3;8:214. doi: 10.3389/fimmu.2017.00214. eCollection 2017. Front Immunol. 2017. PMID: 28316599 Free PMC article.
-
Actions of Retinoic Acid in the Pathophysiology of HIV Infection.Nutrients. 2022 Apr 12;14(8):1611. doi: 10.3390/nu14081611. Nutrients. 2022. PMID: 35458172 Free PMC article. Review.
-
Intestinal dendritic cells in the pathogenesis of inflammatory bowel disease.World J Gastroenterol. 2011 Sep 7;17(33):3761-75. doi: 10.3748/wjg.v17.i33.3761. World J Gastroenterol. 2011. PMID: 21987618 Free PMC article. Review.
References
-
- Bilsborough J, Viney JL. Gastrointestinal dendritic cells play a role in immunity, tolerance, and disease. Gastroenterology. 2004;127:300–309. - PubMed
-
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. - PubMed
-
- Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. - PubMed
-
- Leithäuser F, Trobonjaca Z, Möller P, Reimann J. Clustering of colonic lamina propria CD4(+) T cells to subepithelial dendritic cell aggregates precedes the development of colitis in a murine adoptive transfer model. Lab Invest. 2001;81:1339–4139. - PubMed
-
- Krajina T, Leithäuser F, Möller P, Trobonjaca Z, Reimann J. Colonic lamina propria dendritic cells in mice with CD4+ T cell-induced colitis. Eur J Immunol. 2003;33:1073–1083. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

