Matrix metalloproteinase 2 (MMP2) inhibition: DFT and QM/MM studies of the deprotonation-initialized ring-opening reaction of the sulfoxide analogue of SB-3CT
- PMID: 20039633
- PMCID: PMC2821710
- DOI: 10.1021/jp909327y
Matrix metalloproteinase 2 (MMP2) inhibition: DFT and QM/MM studies of the deprotonation-initialized ring-opening reaction of the sulfoxide analogue of SB-3CT
Abstract
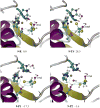
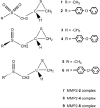
(4-Phenoxyphenylsulfonyl)methylthiirane (SB-3CT) is the selective inhibitor of matrix metalloproteinase 2 (MMP2). The inhibition mechanism of MMP2 by SB-3CT involves C-H deprotonation with concomitant opening of the three-membered heterocycle. In this study, the energetics of the deprotonation-induced ring-opening of (4-phenoxyphenylsulfinyl)methylthiirane, the sulfoxide analogue of SB-3CT, are examined computationally using DFT and QM/MM calculations. A model system, 2-(methylsulfinylmethyl)thiirane, is used to study the stereoelectronic and conformational effects of reaction barriers in methanol. For the model system in methanol solution (using the polarizable continuum model), the reaction barriers range from 17 to 23 kcal/mol with significant stereoelectronic effects. However, the lowest barriers of the (R,R) and (S,R) diastereomers are similar. Two diastereomers of the sulfoxide analogue of SB-3CT are studied in the active site of MMP2 by QM/MM methods with an accurate partial charge fitting procedure. The ring-opening reactions of these two diastereomers have similar reaction energetics. Both are exothermic from the reactant to the ring-opening product (thiolate). The protonation of the thiolate by a water molecule is endothermic in both cases. However, the deprotonation/ring-opening barriers in the MMP2 active site using QM/MM methods for the (R,R) and (S,R) inhibitions are quite different (23.3 and 28.5 kcal/mol, respectively). The TSs identified in QM/MM calculations were confirmed by vibrational frequency analysis and following the reaction path. The (R,R) diastereomer has a hydrogen bond between the sulfoxide oxygen and the backbone NH of Leu191, while the (S,R) has a hydrogen bond between the sulfoxide oxygen and a water molecule. The dissimilar strengths of these hydrogen bonds as well as minor differences in the TS structures contribute to the difference between the barriers. Compared to SB-3CT, both diastereomers of the sulfoxide analogue have higher reaction barriers and have less exothermic reaction energies. This agrees well with the experiments, where SB-3CT is a more effective inhibitor of MMP2 than its sulfoxide analogue.
Figures
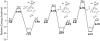

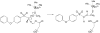

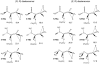
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

