O2-dependent aliphatic carbon-carbon bond cleavage reactivity in a Ni(II) enolate complex having a hydrogen bond donor microenvironment; comparison with a hydrophobic analogue
- PMID: 20039645
- PMCID: PMC2866139
- DOI: 10.1021/ic901981y
O2-dependent aliphatic carbon-carbon bond cleavage reactivity in a Ni(II) enolate complex having a hydrogen bond donor microenvironment; comparison with a hydrophobic analogue
Abstract
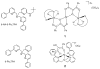
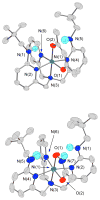
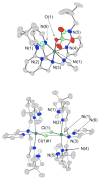
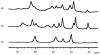
A mononuclear Ni(II) complex having an acireductone type ligand, and supported by the bnpapa (N,N-bis((6-neopentylamino-2-pyridyl)methyl)-N-((2-pyridyl)methyl)amine) ligand, [(bnpapa)Ni(PhC(O)C(OH)C(O)Ph)]ClO(4) (14), has been prepared and characterized by elemental analysis, (1)H NMR, FTIR, and UV-vis. To gain insight into the (1)H NMR features of 14, the air stable analogue complexes [(bnpapa)Ni(CH(3)C(O)CHC(O)CH(3))]ClO(4) (16) and [(bnpapa)Ni(ONHC(O)CH(3))]ClO(4) (17) were prepared and characterized by X-ray crystallography, (1)H NMR, FTIR, UV-vis, mass spectrometry, and solution conductivity measurements. Compounds 16 and 17 are 1:1 electrolyte species in CH(3)CN. (1)H and (2)H NMR studies of 14, 16, and 17 and deuterated analogues revealed that the complexes having six-membered chelate rings for the exogenous ligand (14 and 16) do not have a plane of symmetry within the solvated cation and thus exhibit more complicated (1)H NMR spectra. Compound 17, as well as other simple Ni(II) complexes of the bnpapa ligand (e.g., [(bnpapa)Ni(ClO(4))(CH(3)CN)]ClO(4) (18) and [(bnpapaNi)(2)(mu-Cl)(2)](ClO(4))(2) (19)), exhibit (1)H NMR spectra consistent with the presence of a plane of symmetry within the cation. Treatment of [(bnpapa)Ni(PhC(O)C(OH)C(O)Ph)]ClO(4) (14) with O(2) results in aliphatic carbon-carbon bond cleavage within the acireductone-type ligand and the formation of [(bnpapa)Ni(O(2)CPh)]ClO(4) (9), benzoic acid, benzil, and CO. Use of (18)O(2) in the reaction gives high levels of incorporation (>80%) of one labeled oxygen atom into 9 and benzoic acid. The product mixture and level of (18)O incorporation in this reaction is different than that exhibited by the analogue supported the hydrophobic 6-Ph(2)TPA ligand, [(6-Ph(2)TPA)Ni(PhC(O)C(OH)C(O)Ph)]ClO(4) (2). We propose that this difference is due to variations in the reactivity of bnpapa- and 6-Ph(2)TPA-ligated Ni(II) complexes with triketone and/or peroxide species produced in the reaction pathway.
Figures






References
-
- Pochapsky TC, Ju T, Dang R, Beaulieu R, Pagani GM, OuYang B. In: Metal Ions in Life Sciences. Sigel A, Sigel H, Sigel RKO, editors. Wiley-VCH; Weinheim, Germany: 2007. pp. 473–498.
-
- Mann BE, Motterlini R. Chem Commun. 2007:4197–4208. - PubMed
-
- Motterlini R, Sawle P, Hammad J, Bains S, Alberto R, Foresti R, Green CJ. FASEB J. 2005;19:284–286. - PubMed
-
- Zhang WQ, Atkin AJ, Thatcher RJ, Whitwood AC, Fairlamb IJS, Lynam JM. Dalton Trans. 2009:4351–4358. and references cited therein. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

