The Plasmodium serine-type SERA proteases display distinct expression patterns and non-essential in vivo roles during life cycle progression of the malaria parasite
- PMID: 20039882
- PMCID: PMC2878606
- DOI: 10.1111/j.1462-5822.2009.01419.x
The Plasmodium serine-type SERA proteases display distinct expression patterns and non-essential in vivo roles during life cycle progression of the malaria parasite
Abstract
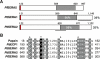
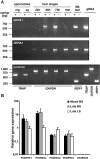
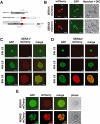
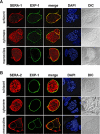
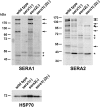
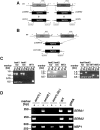
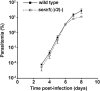
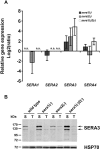
Parasite proteases play key roles in several fundamental steps of the Plasmodium life cycle, including haemoglobin degradation, host cell invasion and parasite egress. Plasmodium exit from infected host cells appears to be mediated by a class of papain-like cysteine proteases called 'serine repeat antigens' (SERAs). A SERA subfamily, represented by Plasmodium falciparum SERA5, contains an atypical active site serine residue instead of a catalytic cysteine. Members of this SERAser subfamily are abundantly expressed in asexual blood stages, rendering them attractive drug and vaccine targets. In this study, we show by antibody localization and in vivo fluorescent tagging with the red fluorescent protein mCherry that the two P. berghei serine-type family members, PbSERA1 and PbSERA2, display differential expression towards the final stages of merozoite formation. Via targeted gene replacement, we generated single and double gene knockouts of the P. berghei SERAser genes. These loss-of-function lines progressed normally through the parasite life cycle, suggesting a specialized, non-vital role for serine-type SERAs in vivo. Parasites lacking PbSERAser showed increased expression of the cysteine-type PbSERA3. Compensatory mechanisms between distinct SERA subfamilies may thus explain the absence of phenotypical defect in SERAser disruptants, and challenge the suitability to develop potent antimalarial drugs based on specific inhibitors of Plasmodium serine-type SERAs.
Figures
Similar articles
-
Evidence for a common role for the serine-type Plasmodium falciparum serine repeat antigen proteases: implications for vaccine and drug design.Infect Immun. 2007 Dec;75(12):5565-74. doi: 10.1128/IAI.00405-07. Epub 2007 Sep 24. Infect Immun. 2007. PMID: 17893128 Free PMC article.
-
Enzymic, phylogenetic, and structural characterization of the unusual papain-like protease domain of Plasmodium falciparum SERA5.J Biol Chem. 2003 Nov 28;278(48):48169-77. doi: 10.1074/jbc.M306755200. Epub 2003 Sep 17. J Biol Chem. 2003. PMID: 13679369
-
Characteristic features of the SERA multigene family in the malaria parasite.Parasit Vectors. 2020 Apr 6;13(1):170. doi: 10.1186/s13071-020-04044-y. Parasit Vectors. 2020. PMID: 32252804 Free PMC article. Review.
-
A Plasmodium cysteine protease required for efficient transition from the liver infection stage.PLoS Pathog. 2020 Sep 21;16(9):e1008891. doi: 10.1371/journal.ppat.1008891. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32956401 Free PMC article.
-
Falcipains and other cysteine proteases of malaria parasites.Adv Exp Med Biol. 2011;712:30-48. doi: 10.1007/978-1-4419-8414-2_3. Adv Exp Med Biol. 2011. PMID: 21660657 Review.
Cited by
-
Malaria parasite pre-erythrocytic infection: preparation meets opportunity.Cell Microbiol. 2012 Mar;14(3):316-24. doi: 10.1111/j.1462-5822.2011.01734.x. Epub 2012 Jan 9. Cell Microbiol. 2012. PMID: 22151703 Free PMC article. Review.
-
A key role for Plasmodium subtilisin-like SUB1 protease in egress of malaria parasites from host hepatocytes.J Biol Chem. 2013 Nov 15;288(46):33336-46. doi: 10.1074/jbc.M113.513234. Epub 2013 Oct 2. J Biol Chem. 2013. PMID: 24089525 Free PMC article.
-
Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound.PLoS One. 2014 Mar 11;9(3):e90972. doi: 10.1371/journal.pone.0090972. eCollection 2014. PLoS One. 2014. PMID: 24618707 Free PMC article.
-
How Should Antibodies against P. falciparum Merozoite Antigens Be Measured?J Trop Med. 2013;2013:493834. doi: 10.1155/2013/493834. Epub 2013 Apr 18. J Trop Med. 2013. PMID: 23690791 Free PMC article.
-
Inhibitory potential of prodomain of Plasmodium falciparum protease serine repeat antigen 5 for asexual blood stages of parasite.PLoS One. 2012;7(1):e30452. doi: 10.1371/journal.pone.0030452. Epub 2012 Jan 24. PLoS One. 2012. PMID: 22291957 Free PMC article.
References
-
- Aoki S, Li J, Itagaki S, Okech BA, Egwang TG, Matsuoka H, et al. Serine repeat antigen (SERA5) is predominantly expressed among the SERA multigene family of Plasmodium falciparum, and the acquired antibody titers correlate with serum inhibition of the parasite growth. J Biol Chem. 2002;277:47533–47540. - PubMed
-
- Arisue N, Hirai M, Arai M, Matsuoka H, Horii T. Phylogeny and evolution of the SERA multigene family in the genus Plasmodium. J Mol E. 2007;65:82–91. - PubMed
-
- Delplace P, Fortier B, Tronchin G, Dubremetz JF, Vernes A. Localization, biosynthesis, processing and isolation of a major 126 kDa antigen of the parasitophorous vacuole of Plasmodium falciparum. Mol Biochem Parasitol. 1987;23:193–201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

