A reproducible brain tumour model established from human glioblastoma biopsies
- PMID: 20040089
- PMCID: PMC2810304
- DOI: 10.1186/1471-2407-9-465
A reproducible brain tumour model established from human glioblastoma biopsies
Abstract
Background: Establishing clinically relevant animal models of glioblastoma multiforme (GBM) remains a challenge, and many commonly used cell line-based models do not recapitulate the invasive growth patterns of patient GBMs. Previously, we have reported the formation of highly invasive tumour xenografts in nude rats from human GBMs. However, implementing tumour models based on primary tissue requires that these models can be sufficiently standardised with consistently high take rates.
Methods: In this work, we collected data on growth kinetics from a material of 29 biopsies xenografted in nude rats, and characterised this model with an emphasis on neuropathological and radiological features.
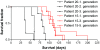
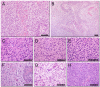
Results: The tumour take rate for xenografted GBM biopsies were 96% and remained close to 100% at subsequent passages in vivo, whereas only one of four lower grade tumours engrafted. Average time from transplantation to the onset of symptoms was 125 days +/- 11.5 SEM. Histologically, the primary xenografts recapitulated the invasive features of the parent tumours while endothelial cell proliferations and necrosis were mostly absent. After 4-5 in vivo passages, the tumours became more vascular with necrotic areas, but also appeared more circumscribed. MRI typically revealed changes related to tumour growth, several months prior to the onset of symptoms.
Conclusions: In vivo passaging of patient GBM biopsies produced tumours representative of the patient tumours, with high take rates and a reproducible disease course. The model provides combinations of angiogenic and invasive phenotypes and represents a good alternative to in vitro propagated cell lines for dissecting mechanisms of brain tumour progression.
Figures





References
-
- Ponten J, Macintyre EH. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74(4):465–486. - PubMed
-
- Schold SC Jr, Friedman HS. Human brain tumor xenografts. Prog Exp Tumor Res. 1984;28:18–31. - PubMed
-
- Mahesparan R, Read TA, Lund-Johansen M, Skaftnesmo KO, Bjerkvig R, Engebraaten O. Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol. 2003;105(1):49–57. - PubMed
-
- Read TA, Farhadi M, Bjerkvig R, Olsen BR, Rokstad AM, Huszthy PC, Vajkoczy P. Intravital microscopy reveals novel antivascular and antitumor effects of endostatin delivered locally by alginate-encapsulated cells. Cancer Res. 2001;61(18):6830–6837. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

