Production and characterization of recombinant pertactin, fimbriae 2 and fimbriae 3 from Bordetella pertussis
- PMID: 20040101
- PMCID: PMC2807877
- DOI: 10.1186/1471-2180-9-274
Production and characterization of recombinant pertactin, fimbriae 2 and fimbriae 3 from Bordetella pertussis
Abstract
Background: Bordetella pertussis is a causative agent of pertussis or whooping cough in humans. Pertactin (Prn), fimbriae 2 (Fim2) and fimbriae 3 (Fim3) of B. pertussis are important virulence factors and immunogens which have been included in some acellular pertussis vaccines. In this present study, we cloned, expressed and purified Prn, Fim2 and Fim3, respectively. The immunogenicity and protective efficacy of the three recombinant proteins (rPrn, rFim2 and rFim3) were investigated in mouse model.
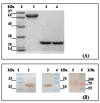
Results: Three recombinant proteins with amount of 12 to 25 mg/L were produced. Compared to the control mice only immunized with adjuvant, serum IgG antibody responses were significantly induced in the mice immunized with rPrn, rFim2 or rFim3 (P < 0.001 for all three proteins). Furthermore, T cell responses characteristic of increased production of IL-2 and TNF-alpha (only for rPrn) were elicited in the mice immunized with the three proteins (P < 0.05 for all three proteins). Immunization with rPrn, but not with rFim2 or rFim3, significantly enhanced clearance of bacteria in the lungs of mice after intranasal challenge with B. pertussis (P < 0.05). When tested in a lethal intracerebral infection model, certain protection was observed in mice immunized with rPrn.
Conclusions: We have developed an efficient method to produce large amounts of rPrn, rFim2, and rFim3 from B. pertussis. The three recombinant proteins induced both humoral and cellular immune responses in mice. Immunization with rPrn also conferred protection against pertussis in mouse infection models. Our results indicated that the recombinant proteins still retain their immunological properties and highlighted the potential of the recombinant proteins for the future development of the B. pertussis vaccines.
Figures
Similar articles
-
Intranasal application of a bifunctional pertactin-RTX fusion antigen elicits protection of mouse airway mucosa against Bordetella pertussis colonization.mSphere. 2025 Apr 29;10(4):e0095924. doi: 10.1128/msphere.00959-24. Epub 2025 Mar 31. mSphere. 2025. PMID: 40162794 Free PMC article.
-
Increasing FIM2/3 antigen-content improves efficacy of Bordetella pertussis vaccines in mice in vivo without altering vaccine-induced human reactogenicity biomarkers in vitro.Vaccine. 2019 Jan 3;37(1):80-89. doi: 10.1016/j.vaccine.2018.11.028. Epub 2018 Nov 23. Vaccine. 2019. PMID: 30478007 Free PMC article.
-
Construction and evaluation of Bordetella pertussis live attenuated vaccine strain BPZE1 producing Fim3.Vaccine. 2018 Mar 7;36(11):1345-1352. doi: 10.1016/j.vaccine.2018.02.017. Epub 2018 Feb 9. Vaccine. 2018. PMID: 29433898
-
Bordetella pertussis and pertactin-deficient clinical isolates: lessons for pertussis vaccines.Expert Rev Vaccines. 2014 Sep;13(9):1135-46. doi: 10.1586/14760584.2014.932254. Epub 2014 Jun 23. Expert Rev Vaccines. 2014. PMID: 24953157 Review.
-
Acellular pertussis vaccines and the role of pertactin and fimbriae.Expert Rev Vaccines. 2007 Feb;6(1):47-56. doi: 10.1586/14760584.6.1.47. Expert Rev Vaccines. 2007. PMID: 17280478 Review.
Cited by
-
Exploiting subtractive genomics to identify novel drug targets and new immunogenic candidates against Bordetella pertussis: an in silico study.Front Bioinform. 2025 May 13;5:1570054. doi: 10.3389/fbinf.2025.1570054. eCollection 2025. Front Bioinform. 2025. PMID: 40433465 Free PMC article.
-
Antibody responses to individual Bordetella pertussis fimbrial antigen Fim2 or Fim3 following immunization with the five-component acellular pertussis vaccine or to pertussis disease.Clin Vaccine Immunol. 2012 Nov;19(11):1776-83. doi: 10.1128/CVI.00355-12. Epub 2012 Sep 5. Clin Vaccine Immunol. 2012. PMID: 22956654 Free PMC article.
-
Expression of Bordetella pertussis Antigens Fused to Different Vectors and Their Effectiveness as Vaccines.Vaccines (Basel). 2021 May 21;9(6):542. doi: 10.3390/vaccines9060542. Vaccines (Basel). 2021. PMID: 34064136 Free PMC article.
-
Immunization with the recombinant Cholera toxin B fused to Fimbria 2 protein protects against Bordetella pertussis infection.Biomed Res Int. 2014;2014:421486. doi: 10.1155/2014/421486. Epub 2014 May 13. Biomed Res Int. 2014. PMID: 24982881 Free PMC article.
References
-
- The world health report 2004-changing history. World Health Organization report. Geneva. 2006.
-
- Zhang XL, Yang ZW, Zhou J, Yu JJ, Wang KA. An Analysis on Current Epidemiological Characteristics of Bordetella pertussis in China. Chin J Vac Immun. 2000;6:93–95.
-
- Pichichero ME, Deloria MA, Rennels MB, Anderson EL, Edwards KM, Decker MD, Englund JA, Steinhoff MC, Deforest A, Meade BD. A safety and immunogenicity comparison of 12 acellular pertussis vaccines and one whole-cell pertussis vaccine given as a fourth dose in 15- to 20-month-old children. Pediatrics. 1997;100:772–788. doi: 10.1542/peds.100.5.772. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

