EBNA3C interacts with Gadd34 and counteracts the unfolded protein response
- PMID: 20040105
- PMCID: PMC2805635
- DOI: 10.1186/1743-422X-6-231
EBNA3C interacts with Gadd34 and counteracts the unfolded protein response
Abstract
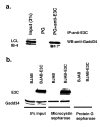
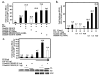
EBNA3C is an EBV-encoded nuclear protein, essential for proliferation of EBV infected B-lymphocytes. Using EBNA3C amino acids 365-545 in a yeast two hybrid screen, we found an interaction with the Growth Arrest and DNA-damage protein, Gadd34. When both proteins are overexpressed, Gadd34 can interact with EBNA3C in both nuclear and cytoplasmic compartments. Amino acids 483-610 of Gadd34, including the two PP1a interaction, and the HSV-1 ICPgamma34.5 homology domains, are required for the interaction. Furthermore, interaction is lost with a mutant of EBNA3C (509 DVIEVID 515-->AVIAVIA), that abolishes EBNA3C coactivation ability as well as SUMO interaction1. In B-cells, Gadd34, and EBNA3C are present in a complex with PP1a using microcystin sepharose affinity purification, Using a lymphoblastoid cell line in which EBNA3C protein levels are conditional on hydroxytamoxifen, surprisingly, we found that (i) EBNA3C maintains phosphorylation of eIF2alpha at serine 51, and (ii) protects against ER stress induced activation of the unfolded protein response as measured by XBP1 (u) versus XBP1(s) protein expression and N-terminal ATF6 cleavage. In reporter assays, overexpression of Gadd34 enhances EBNA3C's ability to co-activate EBNA2 activation of the LMP1 promoter. Collectively the data suggest that EBNA3C interacts with Gadd34, activating the upstream component of the UPR (eIF2alpha phosphorylation) while preventing downstream UPR events (XBP1 activation and ATF6 cleavage).
Figures


Similar articles
-
EBNA3C coactivation with EBNA2 requires a SUMO homology domain.J Virol. 2004 Jan;78(1):367-77. doi: 10.1128/jvi.78.1.367-377.2004. J Virol. 2004. PMID: 14671118 Free PMC article.
-
Epstein-Barr Virus Nuclear Antigen 3 (EBNA3) Proteins Regulate EBNA2 Binding to Distinct RBPJ Genomic Sites.J Virol. 2015 Dec 30;90(6):2906-19. doi: 10.1128/JVI.02737-15. J Virol. 2015. PMID: 26719268 Free PMC article.
-
Modulation of histone acetyltransferase activity through interaction of epstein-barr nuclear antigen 3C with prothymosin alpha.Mol Cell Biol. 2000 Aug;20(15):5722-35. doi: 10.1128/MCB.20.15.5722-5735.2000. Mol Cell Biol. 2000. PMID: 10891508 Free PMC article.
-
Epstein-Barr virus essential antigen EBNA3C attenuates H2AX expression.J Virol. 2014 Apr;88(7):3776-88. doi: 10.1128/JVI.03568-13. Epub 2014 Jan 15. J Virol. 2014. PMID: 24429368 Free PMC article.
-
The Epstein Barr nuclear antigen EBNA3C regulates transcription, cell transformation and cell migration.Front Biosci. 2002 Mar 1;7:d704-16. doi: 10.2741/subraman. Front Biosci. 2002. PMID: 11861219 Review.
Cited by
-
Enterovirus 71 Activates GADD34 via Precursor 3CD to Promote IRES-Mediated Viral Translation.Microbiol Spectr. 2022 Feb 23;10(1):e0138821. doi: 10.1128/spectrum.01388-21. Epub 2022 Jan 5. Microbiol Spectr. 2022. PMID: 34985336 Free PMC article.
-
Exposure to nickel, chromium, or cadmium causes distinct changes in the gene expression patterns of a rat liver derived cell line.PLoS One. 2011;6(11):e27730. doi: 10.1371/journal.pone.0027730. Epub 2011 Nov 16. PLoS One. 2011. PMID: 22110744 Free PMC article.
-
Epstein-Barr virus (EBV) activates NKL homeobox gene HLX in DLBCL.PLoS One. 2019 May 29;14(5):e0216898. doi: 10.1371/journal.pone.0216898. eCollection 2019. PLoS One. 2019. PMID: 31141539 Free PMC article.
-
Association with endoplasmic reticulum promotes proteasomal degradation of GADD34 protein.J Biol Chem. 2011 Jun 17;286(24):21687-96. doi: 10.1074/jbc.M110.212787. Epub 2011 Apr 25. J Biol Chem. 2011. PMID: 21518769 Free PMC article.
-
Role of EBNA-3 Family Proteins in EBV Associated B-cell Lymphomagenesis.Front Microbiol. 2016 Apr 7;7:457. doi: 10.3389/fmicb.2016.00457. eCollection 2016. Front Microbiol. 2016. PMID: 27092119 Free PMC article. Review.
References
-
- Paya CV, Fung JJ, Nalesnik MA, Kieff E, Green M, Gores G, Habermann TM, Wiesner PH, Swinnen JL, Woodle ES, Bromberg JS. Epstein-Barr virus-induced posttransplant lymphoproliferative disorders. ASTS/ASTP EBV-PTLD Task Force and The Mayo Clinic Organized International Consensus Development Meeting. Transplantation. 1999;68(10):1517–1525. doi: 10.1097/00007890-199911270-00015. - DOI - PubMed
-
- Rea D, Delecluse HJ, Hamilton-Dutoit SJ, Marelle L, Joab I, Edelman L, Finet JF, Raphael M. Epstein-Barr virus latent and replicative gene expression in post-transplant lymphoproliferative disorders and AIDS-related non-Hodgkin's lymphomas. French Study Group of Pathology for HIV-associated Tumors. Ann Oncol. 1994;5(Suppl 1):113–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

