Glycosylation-mediated phenylpropanoid partitioning in Populus tremuloides cell cultures
- PMID: 20040108
- PMCID: PMC2808312
- DOI: 10.1186/1471-2229-9-151
Glycosylation-mediated phenylpropanoid partitioning in Populus tremuloides cell cultures
Abstract
Background: Phenylpropanoid-derived phenolic glycosides (PGs) and condensed tannins (CTs) comprise large, multi-purpose non-structural carbon sinks in Populus. A negative correlation between PG and CT concentrations has been observed in several studies. However, the molecular mechanism underlying the relationship is not known.
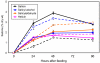
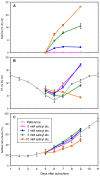
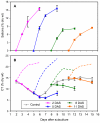
Results: Populus cell cultures produce CTs but not PGs under normal conditions. Feeding salicyl alcohol resulted in accumulation of salicins, the simplest PG, in the cells, but not higher-order PGs. Salicin accrual reflected the stimulation of a glycosylation response which altered a number of metabolic activities. We utilized this suspension cell feeding system as a model for analyzing the possible role of glycosylation in regulating the metabolic competition between PG formation, CT synthesis and growth. Cells accumulated salicins in a dose-dependent manner following salicyl alcohol feeding. Higher feeding levels led to a decrease in cellular CT concentrations (at 5 or 10 mM), and a negative effect on cell growth (at 10 mM). The competition between salicin and CT formation was reciprocal, and depended on the metabolic status of the cells. We analyzed gene expression changes between controls and cells fed with 5 mM salicyl alcohol for 48 hr, a time point when salicin accumulation was near maximum and CT synthesis was reduced, with no effect on growth. Several stress-responsive genes were up-regulated, suggestive of a general stress response in the fed cells. Salicyl alcohol feeding also induced expression of genes associated with sucrose catabolism, glycolysis and the Krebs cycle. Transcript levels of phenylalanine ammonia lyase and most of the flavonoid pathway genes were reduced, consistent with down-regulated CT synthesis.
Conclusions: Exogenous salicyl alcohol was readily glycosylated in Populus cell cultures, a process that altered sugar utilization and phenolic partitioning in the cells. Using this system, we identified candidate genes for glycosyltransferases that may mediate the glycosylation, and for transporters that mediate the subcellular compartmentalization of sugars and phenolic glycosides. The suspension cells appear to represent a facile system for dissecting the regulation of phenolic carbon partitioning, and in turn, its effects on growth in Populus.
Figures




References
-
- Bryant JP, Reichardt PB, Clausen TP, Werner RA. Effects of mineral nutrition on delayed inducible resistance in Alaska paper birch. Ecology. 1993;74:2072–2084. doi: 10.2307/1940853. - DOI
-
- Lavola A. Accumulation of flavonoids and related compounds in birch induced by UV-B irradiance. Tree Physiology. 1998;18:53–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

