Simvastatin enhances hippocampal long-term potentiation in C57BL/6 mice
- PMID: 20040368
- PMCID: PMC2824052
- DOI: 10.1016/j.neuroscience.2009.12.062
Simvastatin enhances hippocampal long-term potentiation in C57BL/6 mice
Abstract
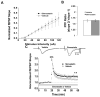
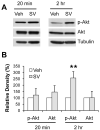
Statins inhibit 3-hydroxy-3-methylglutaryl CoA reductase, the rate-limiting enzyme in the cholesterol biosynthetic pathway, and they are widely used to control plasma cholesterol levels and prevent cardiovascular disease. However, emerging evidence indicates that the beneficial effects of statins extend to the CNS. Statins have been shown to improve the outcome of stroke and traumatic brain injury, and statin use has been associated with a reduced prevalence of Alzheimer's disease (AD) and dementia. However, prospective studies with statins in AD have produced mixed results. Recently, we reported that simvastatin, a widely used statin in humans, enhances learning and memory in non-transgenic mice as well as in transgenic mice with AD-like pathology on a mixed genetic background. However, the cellular and molecular mechanisms underlying the beneficial effects of simvastatin on learning and memory remain elusive. The present study was undertaken to investigate the effect of acute simvastatin treatment on hippocampal long-term potentiation (LTP), a cellular model of learning and memory, in brain slices from C57BL/6 mice. Our results demonstrate that a prolonged in vitro simvastatin treatment for 2-4 h, but not a short-term 20-min exposure, significantly increases the magnitude of LTP at CA3-CA1 synapses without altering basal synaptic transmission or the paired-pulse facilitation ratio in hippocampal slices. Furthermore, we show that phosphorylation of Akt (protein kinase B) is increased significantly in the CA1 region following 2-hour treatment with simvastatin, and that inhibition of Akt phosphorylation suppresses the simvastatin-induced enhancement of LTP. These findings suggest activation of Akt as a molecular pathway for augmented hippocampal LTP by simvastatin treatment, and implicate enhancement of hippocampal LTP as a potential cellular mechanism underlying the beneficial effects of simvastatin on cognitive function.
Copyright (c) 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
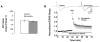

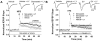
Similar articles
-
Simvastatin-mediated enhancement of long-term potentiation is driven by farnesyl-pyrophosphate depletion and inhibition of farnesylation.Neuroscience. 2012 Jan 27;202:1-9. doi: 10.1016/j.neuroscience.2011.12.007. Epub 2011 Dec 13. Neuroscience. 2012. PMID: 22192838 Free PMC article.
-
Simvastatin Enhances Spatial Memory and Long-Term Potentiation in Hippocampal CA1 via Upregulation of α7 Nicotinic Acetylcholine Receptor.Mol Neurobiol. 2016 Aug;53(6):4060-4072. doi: 10.1007/s12035-015-9344-6. Epub 2015 Jul 22. Mol Neurobiol. 2016. PMID: 26198568
-
Detrimental effects of postnatal exposure to propofol on memory and hippocampal LTP in mice.Brain Res. 2015 Oct 5;1622:321-7. doi: 10.1016/j.brainres.2015.06.044. Epub 2015 Jul 10. Brain Res. 2015. PMID: 26168896
-
Effects of HMG-CoA reductase inhibitors on learning and memory in the guinea pig.Eur J Pharmacol. 2014 Jan 15;723:294-304. doi: 10.1016/j.ejphar.2013.11.018. Epub 2013 Dec 1. Eur J Pharmacol. 2014. PMID: 24296319
-
Sequential activation of soluble guanylate cyclase, protein kinase G and cGMP-degrading phosphodiesterase is necessary for proper induction of long-term potentiation in CA1 of hippocampus. Alterations in hyperammonemia.Neurochem Int. 2004 Nov;45(6):895-901. doi: 10.1016/j.neuint.2004.03.020. Neurochem Int. 2004. PMID: 15312984 Review.
Cited by
-
An acetylcholinesterase inhibitor, eserine, induces long-term depression at CA3-CA1 synapses in the hippocampus of adult rats.J Neurophysiol. 2014 Nov 15;112(10):2388-97. doi: 10.1152/jn.00048.2014. Epub 2014 Aug 20. J Neurophysiol. 2014. PMID: 25143547 Free PMC article.
-
Prenylation of Rho G-proteins: a novel mechanism regulating gene expression and protein stability in human trabecular meshwork cells.Mol Neurobiol. 2012 Aug;46(1):28-40. doi: 10.1007/s12035-012-8249-x. Epub 2012 Mar 7. Mol Neurobiol. 2012. PMID: 22396212
-
Chronic pravastatin but not atorvastatin treatment impairs cognitive function in two rodent models of learning and memory.PLoS One. 2013 Sep 10;8(9):e75467. doi: 10.1371/journal.pone.0075467. eCollection 2013. PLoS One. 2013. PMID: 24040413 Free PMC article.
-
Simvastatin improves learning and memory in control but not in olfactory bulbectomized rats.Psychopharmacology (Berl). 2011 Aug;216(4):537-44. doi: 10.1007/s00213-011-2245-0. Epub 2011 Mar 8. Psychopharmacology (Berl). 2011. PMID: 21384104 Free PMC article.
-
Isoprenoids and protein prenylation: implications in the pathogenesis and therapeutic intervention of Alzheimer's disease.Crit Rev Biochem Mol Biol. 2018 Jun;53(3):279-310. doi: 10.1080/10409238.2018.1458070. Crit Rev Biochem Mol Biol. 2018. PMID: 29718780 Free PMC article. Review.
References
-
- Balduini W, Mazzoni E, Carloni S, De Simoni MG, Perego C, Sironi L, Cimino M. Prophylactic but not delayed administration of simvastatin protects against long-lasting cognitive and morphological consequences of neonatal hypoxic-ischemic brain injury, reduces interleukin-1beta and tumor necrosis factor-alpha mRNA induction, and does not affect endothelial nitric oxide synthase expression. Stroke. 2003;34:2007–2012. - PubMed
-
- Bosel J, Gandor F, Harms C, Synowitz M, Harms U, Djoufack PC, Megow D, Dirnagl U, Hortnagl H, Fink KB, Endres M. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92:1386–1398. - PubMed
-
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. - PubMed
-
- Buxbaum JD, Cullen EI, Friedhoff LT. Pharmacological concentrations of the HMG-CoA reductase inhibitor lovastatin decrease the formation of the Alzheimer beta-amyloid peptide in vitro and in patients. Front Biosci. 2002;7:a50–59. - PubMed
-
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

