A shared mechanism for lipid- and beta-subunit-coordinated stabilization of the activated K+ channel voltage sensor
- PMID: 20040519
- PMCID: PMC2879946
- DOI: 10.1096/fj.09-145219
A shared mechanism for lipid- and beta-subunit-coordinated stabilization of the activated K+ channel voltage sensor
Abstract
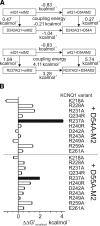
The low-dielectric plasma membrane provides an energy barrier hindering transmembrane movement of charged particles. The positively charged, voltage-sensing fourth transmembrane domain (S4) of voltage-gated ion channels must surmount this energy barrier to initiate channel activation, typically necessitating both membrane depolarization and interaction with membrane lipid phospho-head groups (MLPHGs). In contrast, and despite containing S4, the KCNQ1 K(+) channel alpha subunit exhibits predominantly constitutive activation when in complexes with transmembrane beta subunits, MinK-related peptide (MiRP) 1 (KCNE2) or MiRP2 (KCNE3). Here, using a 2-electrode voltage clamp and scanning mutagenesis of channels heterologously expressed in Xenopus laevis oocytes, we discovered that 2 of the 8 MiRP2 extracellular domain acidic residues (D54 and D55) are important for KCNQ1-MiRP2 constitutive activation. Double-mutant thermodynamic cycle analysis revealed energetic coupling of D54 and D55 to R237 in KCNQ1 S4 but not to 10 other native or introduced polar residues in KCNQ1 S4 and surrounding linkers. MiRP2-D54 and KCNQ1-R237 also similarly dictated susceptibility to the inhibitory effects of MLPHG hydrolysis, whereas other closely situated polar residues did not. Thus, by providing negative charge near the plasma membrane extracellular face, MiRP2 uses a lipomimetic mechanism to constitutively stabilize the activated KCNQ1 voltage sensor.
Figures



Similar articles
-
KCNE1 and KCNE3 modulate KCNQ1 channels by affecting different gating transitions.Proc Natl Acad Sci U S A. 2017 Aug 29;114(35):E7367-E7376. doi: 10.1073/pnas.1710335114. Epub 2017 Aug 14. Proc Natl Acad Sci U S A. 2017. PMID: 28808020 Free PMC article.
-
The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes.J Gen Physiol. 2007 Feb;129(2):121-33. doi: 10.1085/jgp.200609612. Epub 2007 Jan 16. J Gen Physiol. 2007. PMID: 17227916 Free PMC article.
-
KCNE3 acts by promoting voltage sensor activation in KCNQ1.Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):E7286-92. doi: 10.1073/pnas.1516238112. Epub 2015 Dec 14. Proc Natl Acad Sci U S A. 2015. PMID: 26668384 Free PMC article.
-
Interaction of KCNE subunits with the KCNQ1 K+ channel pore.J Physiol. 2006 Feb 1;570(Pt 3):455-67. doi: 10.1113/jphysiol.2005.100644. Epub 2005 Nov 24. J Physiol. 2006. PMID: 16308347 Free PMC article.
-
Sensing voltage across lipid membranes.Nature. 2008 Dec 18;456(7224):891-7. doi: 10.1038/nature07620. Nature. 2008. PMID: 19092925 Free PMC article. Review.
Cited by
-
Ion channel-transporter interactions.Crit Rev Biochem Mol Biol. 2015 Jul-Aug;51(4):257-67. doi: 10.3109/10409238.2016.1172553. Epub 2016 Apr 20. Crit Rev Biochem Mol Biol. 2015. PMID: 27098917 Free PMC article. Review.
-
KCNE1 and KCNE3: The yin and yang of voltage-gated K(+) channel regulation.Gene. 2016 Jan 15;576(1 Pt 1):1-13. doi: 10.1016/j.gene.2015.09.059. Epub 2015 Sep 26. Gene. 2016. PMID: 26410412 Free PMC article. Review.
-
KCNE1 remodels the voltage sensor of Kv7.1 to modulate channel function.Biophys J. 2010 Dec 1;99(11):3599-608. doi: 10.1016/j.bpj.2010.10.018. Biophys J. 2010. PMID: 21112284 Free PMC article.
-
KCNQs: Ligand- and Voltage-Gated Potassium Channels.Front Physiol. 2020 Jun 23;11:583. doi: 10.3389/fphys.2020.00583. eCollection 2020. Front Physiol. 2020. PMID: 32655402 Free PMC article. Review.
-
KCNE1 and KCNE3 modulate KCNQ1 channels by affecting different gating transitions.Proc Natl Acad Sci U S A. 2017 Aug 29;114(35):E7367-E7376. doi: 10.1073/pnas.1710335114. Epub 2017 Aug 14. Proc Natl Acad Sci U S A. 2017. PMID: 28808020 Free PMC article.
References
-
- Long S B, Tao X, Campbell E B, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. - PubMed
-
- Goldstein S A, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. - PubMed
-
- McCrossan Z A, Abbott G W. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. - PubMed
-
- Schroeder B C, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch T J. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

