PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis
- PMID: 20040538
- PMCID: PMC2814515
- DOI: 10.1105/tpc.109.071639
PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis
Abstract
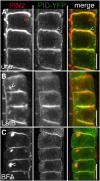
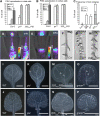
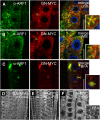
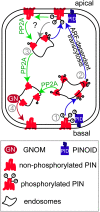
The phytohormone auxin plays a major role in embryonic and postembryonic plant development. The temporal and spatial distribution of auxin largely depends on the subcellular polar localization of members of the PIN-FORMED (PIN) auxin efflux carrier family. The Ser/Thr protein kinase PINOID (PID) catalyzes PIN phosphorylation and crucially contributes to the regulation of apical-basal PIN polarity. The GTP exchange factor on ADP-ribosylation factors (ARF-GEF), GNOM preferentially mediates PIN recycling at the basal side of the cell. Interference with GNOM activity leads to dynamic PIN transcytosis between different sides of the cell. Our genetic, pharmacological, and cell biological approaches illustrate that PID and GNOM influence PIN polarity and plant development in an antagonistic manner and that the PID-dependent PIN phosphorylation results in GNOM-independent polar PIN targeting. The data suggest that PID and the protein phosphatase 2A not only regulate the static PIN polarity, but also act antagonistically on the rate of GNOM-dependent polar PIN transcytosis. We propose a model that includes PID-dependent PIN phosphorylation at the plasma membrane and the subsequent sorting of PIN proteins to a GNOM-independent pathway for polarity alterations during developmental processes, such as lateral root formation and leaf vasculature development.
Figures




Similar articles
-
PIN polarity regulation by AGC-3 kinases and ARF-GEF: a recurrent theme with context dependent modifications for plant development and response.Plant Signal Behav. 2011 Sep;6(9):1333-7. doi: 10.4161/psb.6.9.16611. Plant Signal Behav. 2011. PMID: 21852755 Free PMC article.
-
Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling.Development. 2010 Oct;137(19):3245-55. doi: 10.1242/dev.052456. Development. 2010. PMID: 20823065
-
Dynamic PIN-FORMED auxin efflux carrier phosphorylation at the plasma membrane controls auxin efflux-dependent growth.Proc Natl Acad Sci U S A. 2017 Jan 31;114(5):E887-E896. doi: 10.1073/pnas.1614380114. Epub 2017 Jan 17. Proc Natl Acad Sci U S A. 2017. PMID: 28096328 Free PMC article.
-
Role of the GNOM gene in Arabidopsis apical-basal patterning--From mutant phenotype to cellular mechanism of protein action.Eur J Cell Biol. 2010 Feb-Mar;89(2-3):138-44. doi: 10.1016/j.ejcb.2009.11.020. Epub 2009 Dec 29. Eur J Cell Biol. 2010. PMID: 20036441 Review.
-
Regulation of auxin transport polarity by AGC kinases.Curr Opin Plant Biol. 2008 Oct;11(5):495-502. doi: 10.1016/j.pbi.2008.06.004. Epub 2008 Jul 18. Curr Opin Plant Biol. 2008. PMID: 18640868 Review.
Cited by
-
Transcript profiling of cytokinin action in Arabidopsis roots and shoots discovers largely similar but also organ-specific responses.BMC Plant Biol. 2012 Jul 23;12:112. doi: 10.1186/1471-2229-12-112. BMC Plant Biol. 2012. PMID: 22824128 Free PMC article.
-
Remove, Recycle, Degrade: Regulating Plasma Membrane Protein Accumulation.Plant Cell. 2019 Dec;31(12):2833-2854. doi: 10.1105/tpc.19.00433. Epub 2019 Oct 18. Plant Cell. 2019. PMID: 31628169 Free PMC article. Review.
-
The Protein Phosphatase PP2A Plays Multiple Roles in Plant Development by Regulation of Vesicle Traffic-Facts and Questions.Int J Mol Sci. 2021 Jan 19;22(2):975. doi: 10.3390/ijms22020975. Int J Mol Sci. 2021. PMID: 33478110 Free PMC article. Review.
-
Over 25 years of decrypting PIN-mediated plant development.Nat Commun. 2024 Nov 15;15(1):9904. doi: 10.1038/s41467-024-54240-y. Nat Commun. 2024. PMID: 39548100 Free PMC article. Review.
-
Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane.Mol Syst Biol. 2011 Oct 25;7:540. doi: 10.1038/msb.2011.72. Mol Syst Biol. 2011. PMID: 22027551 Free PMC article.
References
-
- Abas, L., Benjamins, R., Malenica, N., Paciorek, T., Wiśniewska, J., Moulinier-Anzola, J.C., Sieberer, T., Friml, J., and Luschnig, C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8 249–256. - PubMed
-
- Benjamins, R., Quint, A., Weijers, D., Hooykaas, P., and Offringa, R. (2001). The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128 4057–4067. - PubMed
-
- Benková, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertová, D., Jürgens, G., and Friml, J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602. - PubMed
-
- Christensen, S.K., Dagenais, N., Chory, J., and Weigel, D. (2000). Regulation of auxin response by the protein kinase PINOID. Cell 100 469–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

