Auxin response in Arabidopsis under cold stress: underlying molecular mechanisms
- PMID: 20040541
- PMCID: PMC2814496
- DOI: 10.1105/tpc.109.069906
Auxin response in Arabidopsis under cold stress: underlying molecular mechanisms
Abstract
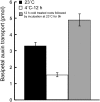
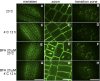
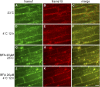
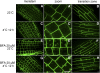
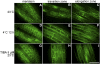
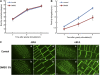
To understand the mechanistic basis of cold temperature stress and the role of the auxin response, we characterized root growth and gravity response of Arabidopsis thaliana after cold stress, finding that 8 to 12 h at 4 degrees C inhibited root growth and gravity response by approximately 50%. The auxin-signaling mutants axr1 and tir1, which show a reduced gravity response, responded to cold treatment like the wild type, suggesting that cold stress affects auxin transport rather than auxin signaling. Consistently, expression analyses of an auxin-responsive marker, IAA2-GUS, and a direct transport assay confirmed that cold inhibits root basipetal (shootward) auxin transport. Microscopy of living cells revealed that trafficking of the auxin efflux carrier PIN2, which acts in basipetal auxin transport, was dramatically reduced by cold. The lateral relocalization of PIN3, which has been suggested to mediate the early phase of root gravity response, was also inhibited by cold stress. Additionally, cold differentially affected various protein trafficking pathways. Furthermore, the inhibition of protein trafficking by cold is independent of cellular actin organization and membrane fluidity. Taken together, these results suggest that the effect of cold stress on auxin is linked to the inhibition of intracellular trafficking of auxin efflux carriers.
Figures
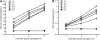
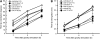



Similar articles
-
Complex regulation of Arabidopsis AGR1/PIN2-mediated root gravitropic response and basipetal auxin transport by cantharidin-sensitive protein phosphatases.Plant J. 2005 Apr;42(2):188-200. doi: 10.1111/j.1365-313X.2005.02369.x. Plant J. 2005. PMID: 15807782
-
Jasmonate modulates endocytosis and plasma membrane accumulation of the Arabidopsis PIN2 protein.New Phytol. 2011 Jul;191(2):360-375. doi: 10.1111/j.1469-8137.2011.03713.x. Epub 2011 Apr 6. New Phytol. 2011. PMID: 21466556
-
Root gravitropism and root hair development constitute coupled developmental responses regulated by auxin homeostasis in the Arabidopsis root apex.New Phytol. 2013 Mar;197(4):1130-1141. doi: 10.1111/nph.12092. Epub 2012 Dec 18. New Phytol. 2013. PMID: 23252740
-
Auxin transport and gravitational research: perspectives.Protoplasma. 2006 Dec;229(2-4):175-81. doi: 10.1007/s00709-006-0216-9. Epub 2006 Dec 16. Protoplasma. 2006. PMID: 17180499 Review.
-
Auxin and Root Gravitropism: Addressing Basic Cellular Processes by Exploiting a Defined Growth Response.Int J Mol Sci. 2021 Mar 9;22(5):2749. doi: 10.3390/ijms22052749. Int J Mol Sci. 2021. PMID: 33803128 Free PMC article. Review.
Cited by
-
DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling.Plant Cell. 2012 May;24(5):1815-33. doi: 10.1105/tpc.112.098707. Epub 2012 May 31. Plant Cell. 2012. PMID: 22652060 Free PMC article.
-
Phosphatidic Acid Directly Regulates PINOID-Dependent Phosphorylation and Activation of the PIN-FORMED2 Auxin Efflux Transporter in Response to Salt Stress.Plant Cell. 2019 Jan;31(1):250-271. doi: 10.1105/tpc.18.00528. Epub 2018 Nov 21. Plant Cell. 2019. PMID: 30464035 Free PMC article.
-
The auxin response factor gene family in banana: genome-wide identification and expression analyses during development, ripening, and abiotic stress.Front Plant Sci. 2015 Sep 15;6:742. doi: 10.3389/fpls.2015.00742. eCollection 2015. Front Plant Sci. 2015. PMID: 26442055 Free PMC article.
-
Genome-wide identification and expression analysis of ClLAX, ClPIN and ClABCB genes families in Citrullus lanatus under various abiotic stresses and grafting.BMC Genet. 2017 Apr 7;18(1):33. doi: 10.1186/s12863-017-0500-z. BMC Genet. 2017. PMID: 28388893 Free PMC article.
-
Protein Levels of Several Arabidopsis Auxin Response Factors Are Regulated by Multiple Factors and ABA Promotes ARF6 Protein Ubiquitination.Int J Mol Sci. 2020 Dec 11;21(24):9437. doi: 10.3390/ijms21249437. Int J Mol Sci. 2020. PMID: 33322385 Free PMC article.
References
-
- Abas, L., Benjamins, R., Malenica, N., Paciorek, T., Wisniewska, J., Moulinier-Anzola, J.C., Sieberer, T., Friml, J., and Luschnig, C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8 249–256. - PubMed
-
- Alonso, A., Queiroz, C.S., and Magalhaes, A.C. (1997). Chilling stress leads to increased cell membrane rigidity in roots of coffee (Coffea arabica L.) seedlings. Biochim. Biophys. Acta 1323 75–84. - PubMed
-
- Baluska, F., and Barlow, P.W. (1993). The role of the microtubular cytoskeleton in determining nuclear chromatin structure and passage of maize root cells through the cell cycle. Eur. J. Cell Biol. 61 160–167. - PubMed
-
- Bannigan, A., Wiedemeier, A.M., Williamson, R.E., Overall, R.L., and Baskin, T.I. (2006). Cortical microtubule arrays lose uniform alignment between cells and are oryzalin resistant in the Arabidopsis mutant, radially swollen 6. Plant Cell Physiol. 47 949–958. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

