Arabidopsis Tic62 and ferredoxin-NADP(H) oxidoreductase form light-regulated complexes that are integrated into the chloroplast redox poise
- PMID: 20040542
- PMCID: PMC2814497
- DOI: 10.1105/tpc.109.069815
Arabidopsis Tic62 and ferredoxin-NADP(H) oxidoreductase form light-regulated complexes that are integrated into the chloroplast redox poise
Abstract
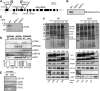
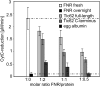
Translocation of nuclear-encoded preproteins across the inner envelope of chloroplasts is catalyzed by the Tic translocon, consisting of Tic110, Tic40, Tic62, Tic55, Tic32, Tic20, and Tic22. Tic62 was proposed to act as a redox sensor of the complex because of its redox-dependent shuttling between envelope and stroma and its specific interaction with the photosynthetic protein ferredoxin-NADP(H) oxidoreductase (FNR). However, the nature of this close relationship so far remained enigmatic. A putative additional localization of Tic62 at the thylakoids mandated further studies examining how this feature might be involved in the respective redox sensing pathway and the interaction with its partner protein. Therefore, both the association with FNR and the physiological role of the third, thylakoid-bound pool of Tic62 were investigated in detail. Coexpression analysis indicates that Tic62 has similar expression patterns as genes involved in photosynthetic functions and protein turnover. At the thylakoids, Tic62 and FNR form high molecular weight complexes that are not involved in photosynthetic electron transfer but are dynamically regulated by light signals and the stromal pH. Structural analyses reveal that Tic62 binds to FNR in a novel binding mode for flavoproteins, with a major contribution from hydrophobic interactions. Moreover, in absence of Tic62, membrane binding and stability of FNR are drastically reduced. We conclude that Tic62 represents a major FNR interaction partner not only at the envelope and in the stroma, but also at the thylakoids of Arabidopsis thaliana and perhaps all flowering plants. Association with Tic62 stabilizes FNR and is involved in its dynamic and light-dependent membrane tethering.
Figures






Similar articles
-
Arabidopsis tic62 trol mutant lacking thylakoid-bound ferredoxin-NADP+ oxidoreductase shows distinct metabolic phenotype.Mol Plant. 2014 Jan;7(1):45-57. doi: 10.1093/mp/sst129. Epub 2013 Sep 16. Mol Plant. 2014. PMID: 24043709
-
Chloroplast-targeted ferredoxin-NADP(+) oxidoreductase (FNR): structure, function and location.Biochim Biophys Acta. 2011 Aug;1807(8):927-34. doi: 10.1016/j.bbabio.2010.10.001. Epub 2010 Oct 8. Biochim Biophys Acta. 2011. PMID: 20934402 Review.
-
Tic62: a protein family from metabolism to protein translocation.BMC Evol Biol. 2007 Mar 20;7:43. doi: 10.1186/1471-2148-7-43. BMC Evol Biol. 2007. PMID: 17374152 Free PMC article.
-
TIC62 redox-regulated translocon composition and dynamics.J Biol Chem. 2008 Mar 14;283(11):6656-67. doi: 10.1074/jbc.M706719200. Epub 2008 Jan 7. J Biol Chem. 2008. PMID: 18180301
-
Protein import into chloroplasts: the Tic complex and its regulation.Biochim Biophys Acta. 2010 Jun;1803(6):740-7. doi: 10.1016/j.bbamcr.2010.01.015. Epub 2010 Jan 25. Biochim Biophys Acta. 2010. PMID: 20100520 Review.
Cited by
-
PBR1 selectively controls biogenesis of photosynthetic complexes by modulating translation of the large chloroplast gene Ycf1 in Arabidopsis.Cell Discov. 2016 May 10;2:16003. doi: 10.1038/celldisc.2016.3. eCollection 2016. Cell Discov. 2016. PMID: 27462450 Free PMC article.
-
Arabidopsis fibrillin 1-2 subfamily members exert their functions via specific protein-protein interactions.J Exp Bot. 2022 Jan 27;73(3):903-914. doi: 10.1093/jxb/erab452. J Exp Bot. 2022. PMID: 34651644 Free PMC article.
-
The Direct Involvement of Dark-Induced Tic55 Protein in Chlorophyll Catabolism and Its Indirect Role in the MYB108-NAC Signaling Pathway during Leaf Senescence in Arabidopsis thaliana.Int J Mol Sci. 2018 Jun 23;19(7):1854. doi: 10.3390/ijms19071854. Int J Mol Sci. 2018. PMID: 29937503 Free PMC article.
-
JASSY, a chloroplast outer membrane protein required for jasmonate biosynthesis.Proc Natl Acad Sci U S A. 2019 May 21;116(21):10568-10575. doi: 10.1073/pnas.1900482116. Epub 2019 May 8. Proc Natl Acad Sci U S A. 2019. PMID: 31068459 Free PMC article.
-
Association of Ferredoxin:NADP+ oxidoreductase with the photosynthetic apparatus modulates electron transfer in Chlamydomonas reinhardtii.Photosynth Res. 2017 Dec;134(3):291-306. doi: 10.1007/s11120-017-0408-5. Epub 2017 Jun 7. Photosynth Res. 2017. PMID: 28593495 Free PMC article.
References
-
- Allahverdiyeva, Y., Mamedov, F., Maenpaa, P., Vass, I., and Aro, E.M. (2005). Modulation of photosynthetic electron transport in the absence of terminal electron acceptors: Characterization of the rbcL deletion mutant of tobacco. Biochim. Biophys. Acta 1709 69–83. - PubMed
-
- Alonso, J.M., et al. (2003). Genome-wide Insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
-
- Andersen, B., Scheller, H.V., and Moller, B.L. (1992). The PSI-E subunit of photosystem I binds ferredoxin:NADP+ oxidoreductase. FEBS Lett. 311 169–173. - PubMed
-
- Aro, E.M., Suorsa, M., Rokka, A., Allahverdiyeva, Y., Paakkarinen, V., Saleem, A., Battchikova, N., and Rintamaki, E. (2005). Dynamics of photosystem II: A proteomic approach to thylakoid protein complexes. J. Exp. Bot. 56 347–356. - PubMed
-
- Aronsson, H., and Jarvis, P. (2002). A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Lett. 529 215–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

