Regulation of the p53 transcriptional response by structurally diverse core promoters
- PMID: 20040571
- PMCID: PMC2807349
- DOI: 10.1101/gad.1856710
Regulation of the p53 transcriptional response by structurally diverse core promoters
Abstract
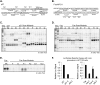
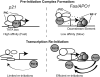
p53 target promoters are structurally diverse and display pronounced differences in RNA polymerase II (RNAP II) occupancy even in unstressed cells, with higher levels observed on cell cycle arrest genes (p21) compared with apoptotic genes (Fas/APO1). This occupancy correlates well with their ability to undergo rapid or delayed stress induction. To understand the basis for such distinct temporal assembly of transcription complexes, we examined the role of core promoter structures in this process. We find that the p21 core promoter directs rapid, TATA box-dependent assembly of RNAP II preinitiation complexes (PICs), but permits few rounds of RNAP II reinitiation. In contrast, PIC formation at the Fas/APO1 core promoter is very inefficient but supports multiple rounds of transcription. We define a downstream element within the Fas/APO1 core promoter that is essential for its activation, and identify nuclear transcription factor Y (NF-Y) as its binding partner. NF-Y acts as a bifunctional transcription factor that regulates basal expression of Fas/APO1 in vivo. Thus, two critical parameters of the stress-induced p53 transcriptional response are the kinetics of gene induction and duration of expression through frequent reinitiation. These features are intrinsic, DNA-encoded properties of diverse core promoters that may be fundamental to anticipatory programming of p53 response genes upon stress.
Figures




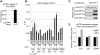
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
