Preferential translation of Hsp83 in Leishmania requires a thermosensitive polypyrimidine-rich element in the 3' UTR and involves scanning of the 5' UTR
- PMID: 20040590
- PMCID: PMC2811665
- DOI: 10.1261/rna.1874710
Preferential translation of Hsp83 in Leishmania requires a thermosensitive polypyrimidine-rich element in the 3' UTR and involves scanning of the 5' UTR
Abstract
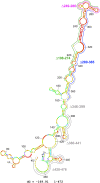
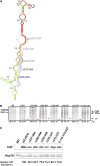
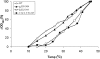
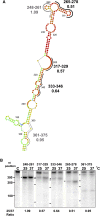
Heat shock proteins (HSPs) provide a useful system for studying developmental patterns in the digenetic Leishmania parasites, since their expression is induced in the mammalian life form. Translation regulation plays a key role in control of protein coding genes in trypanosomatids, and is directed exclusively by elements in the 3' untranslated region (UTR). Using sequential deletions of the Leishmania Hsp83 3' UTR (888 nucleotides [nt]), we mapped a region of 150 nt that was required, but not sufficient for preferential translation of a reporter gene at mammalian-like temperatures, suggesting that changes in RNA structure could be involved. An advanced bioinformatics package for prediction of RNA folding (UNAfold) marked the regulatory region on a highly probable structural arm that includes a polypyrimidine tract (PPT). Mutagenesis of this PPT abrogated completely preferential translation of the fused reporter gene. Furthermore, temperature elevation caused the regulatory region to melt more extensively than the same region that lacked the PPT. We propose that at elevated temperatures the regulatory element in the 3' UTR is more accessible to mediators that promote its interaction with the basal translation components at the 5' end during mRNA circularization. Translation initiation of Hsp83 at all temperatures appears to proceed via scanning of the 5' UTR, since a hairpin structure abolishes expression of a fused reporter gene.
Figures


Similar articles
-
Developmental regulation of heat shock protein 83 in Leishmania. 3' processing and mRNA stability control transcript abundance, and translation id directed by a determinant in the 3'-untranslated region.J Biol Chem. 2001 Dec 21;276(51):47922-9. doi: 10.1074/jbc.M108271200. Epub 2001 Oct 11. J Biol Chem. 2001. PMID: 11598129
-
The expression of HSP83 genes in Leishmania infantum is affected by temperature and by stage-differentiation and is regulated at the levels of mRNA stability and translation.BMC Mol Biol. 2004 Jun 3;5:3. doi: 10.1186/1471-2199-5-3. BMC Mol Biol. 2004. PMID: 15176985 Free PMC article.
-
Regions in the 3' untranslated region confer stage-specific expression to the Leishmania mexicana a600-4 gene.Mol Biochem Parasitol. 2007 Jun;153(2):125-32. doi: 10.1016/j.molbiopara.2007.02.010. Epub 2007 Mar 1. Mol Biochem Parasitol. 2007. PMID: 17433460
-
Lights and shadows on gene organization and regulation of gene expression in Leishmania.Front Biosci (Landmark Ed). 2011 Jun 1;16(6):2069-85. doi: 10.2741/3840. Front Biosci (Landmark Ed). 2011. PMID: 21622163 Review.
-
Regulation of macrophage ceruloplasmin gene expression: one paradigm of 3'-UTR-mediated translational control.Mol Cells. 2005 Oct 31;20(2):167-72. Mol Cells. 2005. PMID: 16267389 Review.
Cited by
-
An RNA thermoswitch regulates daytime growth in Arabidopsis.Nat Plants. 2020 May;6(5):522-532. doi: 10.1038/s41477-020-0633-3. Epub 2020 Apr 13. Nat Plants. 2020. PMID: 32284544 Free PMC article.
-
RNA structure mediated thermoregulation: What can we learn from plants?Front Plant Sci. 2022 Aug 17;13:938570. doi: 10.3389/fpls.2022.938570. eCollection 2022. Front Plant Sci. 2022. PMID: 36092413 Free PMC article. Review.
-
Deletion of a Single LeishIF4E-3 Allele by the CRISPR-Cas9 System Alters Cell Morphology and Infectivity of Leishmania.mSphere. 2019 Sep 4;4(5):e00450-19. doi: 10.1128/mSphere.00450-19. mSphere. 2019. PMID: 31484740 Free PMC article.
-
Post-transcriptional regulation of the trypanosome heat shock response by a zinc finger protein.PLoS Pathog. 2013;9(4):e1003286. doi: 10.1371/journal.ppat.1003286. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23592996 Free PMC article.
-
Novel insights into the Leishmania infantum transcriptome diversity of protein-coding and non-coding sequences in both stages of parasite development using nanopore direct RNA sequencing.BMC Genomics. 2025 Jul 1;26(1):573. doi: 10.1186/s12864-025-11767-8. BMC Genomics. 2025. PMID: 40597600 Free PMC article.
References
-
- Ahmed R, Duncan RF. Translational regulation of Hsp90 mRNA. AUG-proximal 5′-untranslated region elements essential for preferential heat shock translation. J Biol Chem. 2004;279:49919–49930. - PubMed
-
- Argaman M, Aly R, Shapira M. Expression of the heat shock protein 83 in Leishmania is regulated post transcriptionally. Mol Biochem Parasitol. 1994;64:95–110. - PubMed
-
- Boucher N, Wu Y, Dumas C, Dube M, Sereno D, Breton M, Papadopoulou B. A common mechanism of stage-regulated gene expression in Leishmania mediated by a conserved 3′-untranslated region element. J Biol Chem. 2002;277:19511–19520. - PubMed
-
- Bushell M, Stoneley M, Kong YW, Hamilton TL, Spriggs KA, Dobbyn HC, Qin X, Sarnow P, Willis AE. Polypyrimidine tract binding protein regulates IRES-mediated gene expression during apoptosis. Mol Cell. 2006;23:401–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
