TDP-43 is a developmentally regulated protein essential for early embryonic development
- PMID: 20040602
- PMCID: PMC2825476
- DOI: 10.1074/jbc.M109.061846
TDP-43 is a developmentally regulated protein essential for early embryonic development
Erratum in
- J Biol Chem. 2010 Dec 3;285(49):38740
Abstract
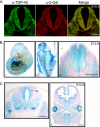
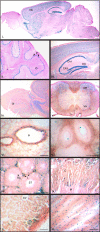
TDP-43 is a DNA/RNA-binding protein implicated in multiple steps of transcriptional and post-transcriptional regulation of gene expression. Alteration of this multifunctional protein is associated with a number of neurodegenerative diseases including amyotrophic lateral sclerosis and frontotemporal lobar degeneration with ubiquitin positive inclusions. Whereas a pathological link to neurodegenerative disorders has been established, the cellular and physiological functions of TDP-43 remain unknown. In this study, we show that TDP-43 is a nuclear protein with persistent high-level expression during embryonic development and with progressively decreased protein levels during postnatal development. In mice where the TDP-43 gene (Tardbp) was disrupted using a gene trap that carries a beta-galactosidase marker gene, heterozygous (Tardbp(+/-)) mice are fertile and healthy, but intercrosses of Tardbp(+/-) mice yielded no viable homozygotic null (Tardbp(-/-)) mice. Indeed, Tardbp(-/-) embryos die between 3.5 and 8.5 days of development. Tardbp(-/-) blastocysts grown in cell culture display abnormal expansion of their inner cell mass. The pattern of beta-galactosidase staining at E9.5 Tardbp(+/-) embryos is predominantly restricted to the neuroepithelium and remains prominent in neural progenitors at E10.5-12.5. TDP-43 is detected in spinal cord progenitors and in differentiated motor neurons as well as in the dorsal root ganglia at E12.5. Beta-galactosidase staining of tissues from adult Tardbp(+/-) mice shows widespread expression of TDP-43, including prominent levels in various regions of the central nervous system afflicted in neurodegenerative disorders. These results indicate that TDP-43 is developmentally regulated and indispensible for early embryonic development.
Figures
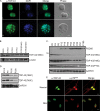
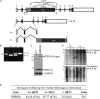
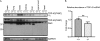
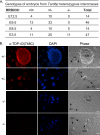

References
-
- Wang H. Y., Wang I. F., Bose J., Shen C. K. (2004) Genomics 83, 130–139 - PubMed
-
- Ayala Y. M., Pantano S., D'Ambrogio A., Buratti E., Brindisi A., Marchetti C., Romano M., Baralle F. E. (2005) J. Mol. Biol. 348, 575–588 - PubMed
-
- Dreyfuss G., Matunis M. J., Piñol-Roma S., Burd C. G. (1993) Annu. Rev. Biochem. 62, 289–321 - PubMed
-
- Buratti E., Baralle F. E. (2008) Front. Biosci. 13, 867–878 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

