Use of the site of subcutaneous insulin administration for the measurement of glucose in patients with type 1 diabetes
- PMID: 20040654
- PMCID: PMC2827515
- DOI: 10.2337/dc09-1532
Use of the site of subcutaneous insulin administration for the measurement of glucose in patients with type 1 diabetes
Abstract
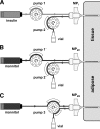
OBJECTIVE To simplify and improve the treatment of patients with type 1 diabetes, we ascertained whether the site of subcutaneous insulin infusion can be used for the measurement of glucose. RESEARCH DESIGN AND METHODS Three special indwelling catheters (24-gauge microperfusion [MP] catheters) were inserted into the subcutaneous adipose tissue of subjects with type 1 diabetes (n = 10; all C-peptide negative). One MP catheter was perfused with short-acting insulin (100 units/ml, Aspart) and used for insulin delivery and simultaneous glucose sampling during an overnight fast and after ingestion of a standard glucose load (75 g). As controls, the further two MP catheters were perfused with an insulin-free solution (5% mannitol) and used for glucose sampling only. Plasma glucose was measured frequently at the bedside. RESULTS Insulin delivery with the MP catheter was adequate to achieve and maintain normoglycemia during fasting and after glucose ingestion. Tissue glucose concentrations derived with the insulin-perfused catheter agreed well with plasma glucose levels. Median correlation coefficient and median absolute relative difference values were found to be 0.93 (interquartile range 0.91-0.97) and 10.9%, respectively. Error grid analysis indicated that the percentage number of tissue values falling in the clinically acceptable range is 99.6%. Comparable analysis results were obtained for the two mannitol-perfused catheters. CONCLUSIONS Our data suggest that estimation of plasma glucose concentrations from the glucose levels directly observed at the site of subcutaneous insulin infusion is feasible and its quality is comparable to that of estimating plasma glucose concentrations from glucose levels measured in insulin-unexposed subcutaneous tissue.
Figures

Similar articles
-
Periodic extraction of interstitial fluid from the site of subcutaneous insulin infusion for the measurement of glucose: a novel single-port technique for the treatment of type 1 diabetes patients.Diabetes Technol Ther. 2013 Jan;15(1):50-9. doi: 10.1089/dia.2012.0173. Epub 2012 Nov 5. Diabetes Technol Ther. 2013. PMID: 23126579 Free PMC article.
-
Glucose levels at the site of subcutaneous insulin administration and their relationship to plasma levels.Diabetes Care. 2010 Apr;33(4):833-8. doi: 10.2337/dc09-1531. Epub 2010 Jan 22. Diabetes Care. 2010. PMID: 20097778 Free PMC article. Clinical Trial.
-
Accuracy assessment of online glucose monitoring by a subcutaneous enzymatic glucose sensor during exercise in patients with type 1 diabetes treated by continuous subcutaneous insulin infusion.Diabetes Metab. 2013 May;39(3):258-62. doi: 10.1016/j.diabet.2012.12.004. Epub 2013 Mar 19. Diabetes Metab. 2013. PMID: 23522730
-
Tissue Response to Subcutaneous Infusion Catheter.J Diabetes Sci Technol. 2020 Mar;14(2):226-232. doi: 10.1177/1932296819837972. Epub 2019 Mar 31. J Diabetes Sci Technol. 2020. PMID: 30931603 Free PMC article.
-
Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics.Diabetes Metab Res Rev. 2016 Jan;32(1):21-39. doi: 10.1002/dmrr.2653. Epub 2015 Jun 22. Diabetes Metab Res Rev. 2016. PMID: 25865292 Free PMC article. Review.
Cited by
-
A Glucose-Responsive Cannula for Automated and Electronics-Free Insulin Delivery.Adv Mater. 2024 Jul;36(27):e2403594. doi: 10.1002/adma.202403594. Epub 2024 Apr 29. Adv Mater. 2024. PMID: 38639424 Free PMC article.
-
Measuring glucose at the site of insulin delivery with a redox-mediated sensor.Biosens Bioelectron. 2020 Oct 1;165:112221. doi: 10.1016/j.bios.2020.112221. Epub 2020 Apr 29. Biosens Bioelectron. 2020. PMID: 32729464 Free PMC article.
-
Can glucose be monitored accurately at the site of subcutaneous insulin delivery?J Diabetes Sci Technol. 2014 May;8(3):568-74. doi: 10.1177/1932296814522805. Epub 2014 Feb 18. J Diabetes Sci Technol. 2014. PMID: 24876621 Free PMC article. Review.
-
Combining an Electrochemical Continuous Glucose Sensor With an Insulin Delivery Cannula: A Feasibility Study.J Diabetes Sci Technol. 2024 Nov;18(6):1273-1280. doi: 10.1177/19322968241236771. Epub 2024 Mar 16. J Diabetes Sci Technol. 2024. PMID: 38491800 Free PMC article.
-
Moving Toward a Unified Platform for Insulin Delivery and Sensing of Inputs Relevant to an Artificial Pancreas.J Diabetes Sci Technol. 2017 Mar;11(2):308-314. doi: 10.1177/1932296816682762. Epub 2016 Dec 13. J Diabetes Sci Technol. 2017. PMID: 28264192 Free PMC article. Review.
References
-
- Daneman D: Type 1 diabetes. Lancet 2006; 367: 847– 858 - PubMed
-
- Shalitin S, Phillip M: The role of new technologies in treating children and adolescents with type 1 diabetes mellitus. Pediatric Diabetes 2007; 8 ( Suppl. 6): 72– 79 - PubMed
-
- Lindpointner S, Korsatko S, Köhler G, Köhler H, Ellmerer M, Schaupp L, Pieber TR, Regittnig W: Glucose concentration at the subcutaneous site of insulin delivery: effect of variable insulin infusion rates. Abstract presented at the 2nd European Diabetes Technology and Transplantation Meeting, 27–29 January 2008, Innsbruck, Austria
-
- Schaupp L, Ellmerer M, Brunner GA, Wutte A, Sendlhofer G, Trajanoski Z, Skrabal F, Pieber TR, Wach P: Direct access to interstitial fluid in adipose tissue in humans by use of open-flow microperfusion. Am J Physiol 1999; 276: E401– E408 - PubMed
-
- Trajanoski Z, Brunner GA, Schaupp L, Ellmerer M, Wach P, Pieber TR, Kotanko P, Skrabal F: Open-flow microperfusion of subcutaneous adipose tissue for on-line continuous ex vivo measurement of glucose concentration. Diabetes Care 1997; 20: 1114– 1121 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

