Intrathymic transplantation of bone marrow-derived progenitors provides long-term thymopoiesis
- PMID: 20040762
- PMCID: PMC2837328
- DOI: 10.1182/blood-2009-06-229724
Intrathymic transplantation of bone marrow-derived progenitors provides long-term thymopoiesis
Abstract
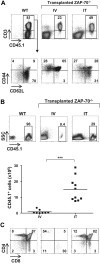
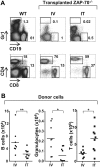
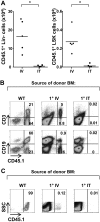
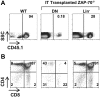
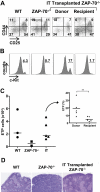
The sustained differentiation of T cells in the thymus cannot be maintained by resident intrathymic (IT) precursors and requires that progenitors be replenished from the bone marrow (BM). In patients with severe combined immunodeficiency (SCID) treated by hematopoietic stem cell transplantation, late T-cell differentiation defects are thought to be due to an insufficient entry of donor BM progenitors into the thymus. Indeed, we find that the intravenous injection of BM progenitors into nonconditioned zeta-chain-associated protein kinase 70 (ZAP-70)-deficient mice with SCID supports short- but not long-term thymopoiesis. Remarkably, we now show that the IT administration of these progenitors produces a significant level of donor-derived thymopoiesis for more than 6 months after transplantation. In contrast to physiologic thymopoiesis, long-term donor thymopoiesis was not due to the continued recruitment of progenitors from the BM. Rather, IT transplantation resulted in the unique generation of a large population of early c-Kit(high) donor precursors within the thymus. These ZAP-70-deficient mice that received an IT transplant had a significantly increased prothymocyte niche compared with their untreated counterparts; this phenotype was associated with the generation of a medulla. Thus, IT administration of BM progenitors results in the filling of an expanded precursor niche and may represent a strategy for enhancing T-cell differentiation in patients with SCID.
Figures

Similar articles
-
Intrathymic progenitor cell transplantation across histocompatibility barriers results in the persistence of early thymic progenitors and T-cell differentiation.Blood. 2013 Mar 14;121(11):2144-53. doi: 10.1182/blood-2012-08-447417. Epub 2013 Jan 10. Blood. 2013. PMID: 23305740 Free PMC article.
-
Dynamics of human prothymocytes and xenogeneic thymopoiesis in hematopoietic stem cell-engrafted nonobese diabetic-SCID/IL-2rγnull mice.J Immunol. 2012 Aug 15;189(4):1648-60. doi: 10.4049/jimmunol.1201251. Epub 2012 Jul 13. J Immunol. 2012. PMID: 22798679
-
Stem cell factor consistently improves thymopoiesis after experimental transplantation of murine or human hematopoietic stem cells in immunodeficient mice.J Immunol. 2011 Sep 15;187(6):2974-81. doi: 10.4049/jimmunol.1004209. Epub 2011 Aug 22. J Immunol. 2011. PMID: 21859956
-
Concise review: hematopoietic stem cell transplantation: targeting the thymus.Stem Cells. 2013 Jul;31(7):1245-51. doi: 10.1002/stem.1378. Stem Cells. 2013. PMID: 23554173 Review.
-
Thymus Colonization: Who, How, How Many?Arch Immunol Ther Exp (Warsz). 2018 Apr;66(2):81-88. doi: 10.1007/s00005-017-0503-5. Epub 2017 Dec 29. Arch Immunol Ther Exp (Warsz). 2018. PMID: 29288431 Review.
Cited by
-
Bone marrow-derived IL-13Rα1-positive thymic progenitors are restricted to the myeloid lineage.J Immunol. 2012 Apr 1;188(7):3208-16. doi: 10.4049/jimmunol.1103316. Epub 2012 Feb 20. J Immunol. 2012. PMID: 22351937 Free PMC article.
-
Limiting Thymic Precursor Supply Increases the Risk of Lymphoid Malignancy in Murine X-Linked Severe Combined Immunodeficiency.Mol Ther Nucleic Acids. 2017 Mar 17;6:1-14. doi: 10.1016/j.omtn.2016.11.011. Epub 2016 Dec 10. Mol Ther Nucleic Acids. 2017. PMID: 28325276 Free PMC article.
-
Free-hand ultrasound guidance permits safe and efficient minimally invasive intrathymic injections in both young and aged mice.Ultrasound Med Biol. 2015 Apr;41(4):1105-11. doi: 10.1016/j.ultrasmedbio.2014.11.011. Epub 2015 Feb 17. Ultrasound Med Biol. 2015. PMID: 25701534 Free PMC article.
-
Intrathymic adeno-associated virus gene transfer rapidly restores thymic function and long-term persistence of gene-corrected T cells.J Allergy Clin Immunol. 2020 Feb;145(2):679-697.e5. doi: 10.1016/j.jaci.2019.08.029. Epub 2019 Sep 9. J Allergy Clin Immunol. 2020. PMID: 31513879 Free PMC article.
-
Intrathymic progenitor cell transplantation across histocompatibility barriers results in the persistence of early thymic progenitors and T-cell differentiation.Blood. 2013 Mar 14;121(11):2144-53. doi: 10.1182/blood-2012-08-447417. Epub 2013 Jan 10. Blood. 2013. PMID: 23305740 Free PMC article.
References
-
- Spangrude GJ, Weissman IL. Mature T cells generated from single thymic clones are phenotypically and functionally heterogeneous. J Immunol. 1988;141(6):1877–1890. - PubMed
-
- Scollay R, Smith J, Stauffer V. Dynamics of early T cells: prothymocyte migration and proliferation in the adult mouse thymus. Immunol Rev. 1986;91:129–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

