Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB)
- PMID: 20040913
- PMCID: PMC2831133
- DOI: 10.1038/mt.2009.292
Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB)
Erratum in
-
Corrigendum to "Directed Evolution of a Novel Adeno-associated Virus (AAV) Vector That Crosses the Seizure-compromised Blood-Brain Barrier (BBB)".Mol Ther. 2010 May;18(5):1054. doi: 10.1038/mt.2010.6. Epub 2016 Dec 6. Mol Ther. 2010. PMID: 28178554 Free PMC article. No abstract available.
Abstract
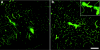
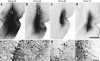
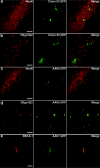
DNA shuffling and directed evolution were employed to develop a novel adeno-associated virus (AAV) vector capable of crossing the seizure-compromised blood-brain barrier (BBB) and transducing cells in the brain. Capsid DNA from AAV serotypes 1-6, 8, and 9 were shuffled and recombined to create a library of chimeric AAVs. One day after kainic acid-induced limbic seizure activity in rats, the virus library was infused intravenously (i.v.), and 3 days later, neuron-rich cells were mechanically dissociated from seizure-sensitive brain sites, collected and viral DNA extracted. After three cycles of selection, green fluorescent protein (GFP)-packaged clones were administered directly into brain or i.v. 1 day after kainic acid-induced seizures. Several clones that were effective after intracranial administration did not transduce brain cells after the i.v. administration. However, two clones (32 and 83) transduced the cells after direct brain infusion and after i.v. administration transduced the cells that were localized to the piriform cortex and ventral hippocampus, areas exhibiting a seizure-compromised BBB. No transduction occurred in areas devoid of BBB compromise. Only one parental serotype (AAV8) exhibited a similar expression profile, but the biodistribution of 32 and 83 diverged dramatically from this parental serotype. Thus, novel AAV vectors have been created that can selectively cross the seizure-compromised BBB and transduce cells.
Figures




References
-
- Haberman RP, Samulski RJ., and , McCown TJ. Attenuation of seizures and neuronal death by adeno-associated virus vector galanin expression and secretion. Nat Med. 2003;9:1076–1080. - PubMed
-
- McCown TJ. Adeno-associated virus-mediated expression and constitutive secretion of galanin suppresses limbic seizure activity in vivo. Mol Ther. 2006;14:63–68. - PubMed
-
- Foti SB, Russek ST, Brooks-Kayal AR., and , McCown TJ.2009Viral vector gene therapy for epilepsyIn: Baraban, SC (ed). Animal Models of Epilepsy: Methods and Innovations Humana Press: New York; 235–250.
-
- Maheshri N, Koerber JT, Kaspar BK., and , Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotechnol. 2006;24:198–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

