Glial dysfunction in abstinent methamphetamine abusers
- PMID: 20040926
- PMCID: PMC2949186
- DOI: 10.1038/jcbfm.2009.261
Glial dysfunction in abstinent methamphetamine abusers
Abstract
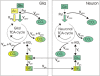
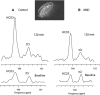
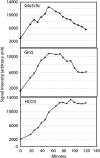
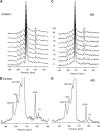
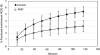
Persistent neurochemical abnormalities in frontal brain structures are believed to result from methamphetamine use. We developed a localized (13)C magnetic resonance spectroscopy (MRS) assay on a conventional MR scanner, to quantify selectively glial metabolic flux rate in frontal brain of normal subjects and a cohort of recovering abstinent methamphetamine abusers. Steady-state bicarbonate concentrations were similar, between 11 and 15 mmol/L in mixed gray-white matter of frontal brain of normal volunteers and recovering methamphetamine-abusing subjects (P>0.1). However, glial (13)C-bicarbonate production rate from [1-(13)C]acetate, equating with glial tricarboxylic acid (TCA) cycle rate, was significantly reduced in frontal brain of abstinent methamphetamine-addicted women (methamphetamine 0.04 micromol/g per min (N=5) versus controls 0.11 micromol/g per min (N=5), P=0.001). This is equivalent to 36% of the normal glial TCA cycle rate. Severe reduction in glial TCA cycle rate that normally comprises 10% of total cerebral metabolic rate may impact operation of the neuronal glial glutamate cycle and result in accumulation of frontal brain glutamate, as observed in these recovering methamphetamine abusers. Although these are the first studies to define directly an abnormality in glial metabolism in human methamphetamine abuse, sequential studies using analogous (13)C MRS methods may determine 'cause and effect' between glial failure and neuronal injury.
Figures
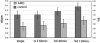
Similar articles
-
Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study.Drug Alcohol Depend. 2007 Apr 17;88(1):28-35. doi: 10.1016/j.drugalcdep.2006.09.011. Epub 2006 Nov 7. Drug Alcohol Depend. 2007. PMID: 17084995
-
Tricarboxylic acid cycle of glia in the in vivo human brain.NMR Biomed. 2002 Feb;15(1):1-5. doi: 10.1002/nbm.725. NMR Biomed. 2002. PMID: 11840547
-
Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities.Am J Psychiatry. 2005 Feb;162(2):361-9. doi: 10.1176/appi.ajp.162.2.361. Am J Psychiatry. 2005. PMID: 15677602 Free PMC article.
-
Reflections on the application of 13C-MRS to research on brain metabolism.NMR Biomed. 2003 Oct-Nov;16(6-7):303-12. doi: 10.1002/nbm.844. NMR Biomed. 2003. PMID: 14679497 Review.
-
Regulation of glial metabolism studied by 13C-NMR.NMR Biomed. 2003 Oct-Nov;16(6-7):370-99. doi: 10.1002/nbm.850. NMR Biomed. 2003. PMID: 14679501 Review.
Cited by
-
Decreased frontal lobe phosphocreatine levels in methamphetamine users.Drug Alcohol Depend. 2013 Apr 1;129(1-2):102-9. doi: 10.1016/j.drugalcdep.2012.09.015. Epub 2012 Oct 18. Drug Alcohol Depend. 2013. PMID: 23084413 Free PMC article.
-
An Update of the Review of Neuropsychological Consequences of HIV and Substance Abuse: A Literature Review and Implications for Treatment and Future Research.Curr Drug Abuse Rev. 2015;8(1):50-71. doi: 10.2174/1874473708666150309124820. Curr Drug Abuse Rev. 2015. PMID: 25751583 Free PMC article. Review.
-
Glutamate and glutamine: a review of in vivo MRS in the human brain.NMR Biomed. 2013 Dec;26(12):1630-46. doi: 10.1002/nbm.3045. Epub 2013 Oct 4. NMR Biomed. 2013. PMID: 24123328 Free PMC article. Review.
-
Methamphetamine addiction: involvement of CREB and neuroinflammatory signaling pathways.Psychopharmacology (Berl). 2016 May;233(10):1945-62. doi: 10.1007/s00213-016-4235-8. Epub 2016 Feb 12. Psychopharmacology (Berl). 2016. PMID: 26873080 Free PMC article. Review.
-
The Effect of Chronic Methamphetamine Exposure on the Hippocampal and Olfactory Bulb Neuroproteomes of Rats.PLoS One. 2016 Apr 15;11(4):e0151034. doi: 10.1371/journal.pone.0151034. eCollection 2016. PLoS One. 2016. PMID: 27082425 Free PMC article.
References
-
- Abulseoud O, Sailasuta N, Hernandez M, Ross BD. Impact of depression on cerebral and cognitive function in abstinent methamphetamine users. Proc Int Soc Magn Reson Med. 2009;17:210.
-
- Agabiti E, Todisco T, Grassi V, Sorbini CA. Calculation of plasma bicarbonates using Henderson–Hasselbach equation. Influence of pK changes as a function of pH. Minerva Nefrol. 1973;20:23–27. - PubMed
-
- Bachelard H. Landmarks in the application of 13C-magnetic resonance spectroscopy to studies of neuronal/glial relationships. Dev Neurosci. 1998;20:277–288. - PubMed
-
- Bluml S, Moreno-Torres A, Ross BD. [1-13C]glucose MRS in chronic hepatic encephalopathy in man. Magn Reson Med. 2001;45:981–993. - PubMed
-
- Bluml S, Moreno-Torres A, Shic F, Nguy CH, Ross BD. Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed. 2002;15:1–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

