Translational studies of alcoholism: bridging the gap
- PMID: 20041042
- PMCID: PMC2798743
Translational studies of alcoholism: bridging the gap
Abstract
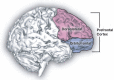
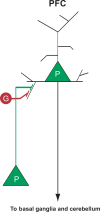
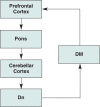
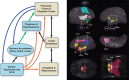
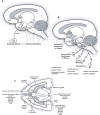
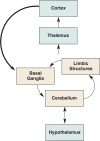
Human studies are necessary to identify and classify the brain systems predisposing individuals to develop alcohol use disorders and those modified by alcohol, while animal models of alcoholism are essential for a mechanistic understanding of how chronic voluntary alcohol consumption becomes compulsive, how brain systems become damaged, and how damage resolves. Our current knowledge of the neuroscience of alcohol dependence has evolved from the interchange of information gathered from both human alcoholics and animal models of alcoholism. Together, studies in humans and animal models have provided support for the involvement of specific brain structures over the course of alcohol addiction, including the prefrontal cortex, basal ganglia, cerebellum, amygdala, hippocampus, and the hypothalamic-pituitary-adrenal axis.
Keywords: Alcohol dependence; alcohol and other drug effects and consequences; alcoholism; animal models; animal studies; brain; chronic alcohol exposure; environmental factors; genetic factors; human studies; neurobiology; translational studies.
Figures

References
-
- Abi-Dargham A, Krystal JH, Anjilvel S, et al. Alterations of benzodiazepine receptors in type II alcoholic subjects measured with SPECT and [123I]iomazenil. American Journal of Psychiatry. 1998;155:1550–1555. - PubMed
-
- Adalsteinsson E, Sullivan EV, Pfefferbaum A. Biochemical, functional and microstructural magnetic resonance imaging (MRI) In: Liu Y, Lovinger DM, editors. Methods in Alcohol-Related Neuroscience Research. Boca Raton, FL: CRC Press; 2002. pp. 345–372.
-
- Adams KM, Gilman S, Koeppe R, et al. Correlation of neuropsychological function with cerebral metabolic rate in subdivisions of the frontal lobes of older alcoholic patients measured with [18F]fluorodeoxyglucose and positron emission tomography. Neuropsychology. 1995;9:275–280.
-
- Adinoff B, Martin PR, Bone GH, et al. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Archives of General Psychiatry. 1990;47:325–330. - PubMed
-
- Afifi AK, Bergman RA. Functional Neuroanatomy: Text and Atlas. New York: McGraw-Hill; 1998.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
