Yersiniabactin reduces the respiratory oxidative stress response of innate immune cells
- PMID: 20041108
- PMCID: PMC2795162
- DOI: 10.1371/journal.pone.0008240
Yersiniabactin reduces the respiratory oxidative stress response of innate immune cells
Abstract
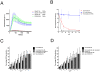
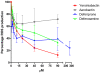
Enterobacteriaceae that contain the High Pathogenicity Island (HPI), which encodes the siderophore yersiniabactin, display increased virulence. This increased virulence may be explained by the increased iron scavenging of the bacteria, which would both enhance bacterial growth and limit the availability of iron to cells of the innate immune system, which require iron to catalyze the Haber-Weiss reaction that produces hydroxyl radicals. In this study, we show that yersiniabactin increases bacterial growth when iron-saturated lactoferrin is the main iron source. This suggests that yersiniabactin provides bacteria with additional iron from saturated lactoferrin during infection. Furthermore, the production of ROS by polymorphonuclear leukocytes, monocytes, and a mouse macrophage cell line is blocked by yersiniabactin, as yersiniabactin reduces iron availability to the cells. Importantly, iron functions as a catalyst during the Haber-Weiss reaction, which generates hydroxyl radicals. While the physiologic role of the Haber-Weiss reaction in the production of hydroxyl radicals has been controversial, the siderophores yersiniabactin, aerobactin, and deferoxamine and the iron-chelator deferiprone also reduce ROS production in activated innate immune cells. This suggests that this reaction takes place under physiological conditions. Of the tested iron chelators, yersiniabactin was the most effective in reducing the ROS production in the tested innate immune cells. The likely decreased bacterial killing by innate immune cells resulting from the reduced production of hydroxyl radicals may explain why the HPI-containing Enterobacteriaceae are more virulent. This model centered on the reduced killing capacity of innate immune cells, which is indirectly caused by yersiniabactin, is in agreement with the observation that the highly pathogenic group of Yersinia is more lethal than the weakly pathogenic and the non-pathogenic group.
Conflict of interest statement
Figures


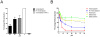
References
-
- Senior K. Yersinia pestis: a force to be reckoned with. Lancet Infect Dis. 2008;8:746.
-
- Prentice MB, Rahalison L. Plague. Lancet. 2007;369:1196–1207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

