The influence of recombinant production on the immunologic behavior of birch pollen isoallergens
- PMID: 20041109
- PMCID: PMC2795169
- DOI: 10.1371/journal.pone.0008457
The influence of recombinant production on the immunologic behavior of birch pollen isoallergens
Abstract
Background: Allergic reactions towards the birch major pollen allergen Bet v 1 are among the most common causes of spring pollinosis in the temperate climate zone of the Northern hemisphere. Natural Bet v 1 is composed of a complex mixture of different isoforms. Detailed analysis of recombinant Bet v 1 isoforms revealed striking differences in immunologic as well as allergenic properties of the molecules, leading to a classification of Bet v 1 isoforms into high, medium, and low IgE binding proteins. Especially low IgE binding Bet v 1 isoforms have been described as ideal candidates for desensitizing allergic patients with allergen specific immunotherapy (SIT). Since diagnosis and therapy of allergic diseases are highly dependent on recombinant proteins, continuous improvement of protein production is an absolute necessity.
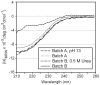
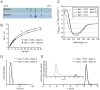
Methodology: Therefore, two different methods for recombinant production of a low IgE binding Bet v 1 isoform were applied; one based on published protocols, the other by implementing latest innovations in protein production. Both batches of Bet v 1.0401 were extensively characterized by an array of physicochemical as well as immunological methods to compare protein primary structure, purity, quantity, folding, aggregation state, thermal stability, and antibody binding capacity.
Conclusion: The experiments demonstrated that IgE antibody binding properties of recombinant isoallergens can be significantly influenced by the production method directly affecting possible clinical applications of the molecules.
Conflict of interest statement
Figures
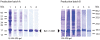
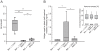
Similar articles
-
Dissection of immunoglobulin E and T lymphocyte reactivity of isoforms of the major birch pollen allergen Bet v 1: potential use of hypoallergenic isoforms for immunotherapy.J Exp Med. 1996 Feb 1;183(2):599-609. doi: 10.1084/jem.183.2.599. J Exp Med. 1996. PMID: 8627171 Free PMC article.
-
Bet v 1--a Trojan horse for small ligands boosting allergic sensitization?Clin Exp Allergy. 2014 Aug;44(8):1083-93. doi: 10.1111/cea.12361. Clin Exp Allergy. 2014. PMID: 24979350
-
Pollen-related food allergy: cloning and immunological analysis of isoforms and mutants of Mal d 1, the major apple allergen, and Bet v 1, the major birch pollen allergen.Eur J Nutr. 1999 Aug;38(4):201-15. doi: 10.1007/s003940050063. Eur J Nutr. 1999. PMID: 10502033
-
Characterization of wild-type recombinant Bet v 1a as a candidate vaccine against birch pollen allergy.Int Arch Allergy Immunol. 2005 Mar;136(3):239-49. doi: 10.1159/000083950. Epub 2005 Feb 16. Int Arch Allergy Immunol. 2005. PMID: 15722633
-
Ligand Binding of PR-10 Proteins with a Particular Focus on the Bet v 1 Allergen Family.Curr Allergy Asthma Rep. 2020 May 19;20(7):25. doi: 10.1007/s11882-020-00918-4. Curr Allergy Asthma Rep. 2020. PMID: 32430735 Free PMC article. Review.
Cited by
-
The dichotomy of pathogens and allergens in vaccination approaches.Front Microbiol. 2014 Jul 16;5:365. doi: 10.3389/fmicb.2014.00365. eCollection 2014. Front Microbiol. 2014. PMID: 25076945 Free PMC article. Review.
-
Tackling Bet v 1 and associated food allergies with a single hybrid protein.J Allergy Clin Immunol. 2017 Aug;140(2):525-533.e10. doi: 10.1016/j.jaci.2016.09.055. Epub 2016 Dec 7. J Allergy Clin Immunol. 2017. PMID: 27939703 Free PMC article.
-
Conformational Flexibility Differentiates Naturally Occurring Bet v 1 Isoforms.Int J Mol Sci. 2017 Jun 3;18(6):1192. doi: 10.3390/ijms18061192. Int J Mol Sci. 2017. PMID: 28587205 Free PMC article.
-
Reshaping the Bet v 1 fold modulates T(H) polarization.J Allergy Clin Immunol. 2011 Jun;127(6):1571-8.e9. doi: 10.1016/j.jaci.2011.01.064. Epub 2011 Mar 21. J Allergy Clin Immunol. 2011. PMID: 21420160 Free PMC article.
-
Structural Alterations of Antigens at the Material Interface: An Early Decision Toolbox Facilitating Safe-by-Design Nanovaccine Development.Int J Mol Sci. 2021 Oct 8;22(19):10895. doi: 10.3390/ijms221910895. Int J Mol Sci. 2021. PMID: 34639235 Free PMC article.
References
-
- Kay AB. Allergy and allergic diseases. Second of two parts. N Engl J Med. 2001;344:109–113. - PubMed
-
- Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344:30–37. - PubMed
-
- Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. - PubMed
-
- Wallner M, Briza P, Thalhamer J, Ferreira F. Specific immunotherapy in pollen allergy. Curr Opin Mol Ther. 2007;9:160–167. - PubMed
-
- International Union of Immunological Society, WHO sub-committee for allergen nomenclature. Available: www.allergen.org. Accessed 2009 Dec 08.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

