Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review
- PMID: 20041113
- PMCID: PMC2795193
- DOI: 10.1371/journal.pone.0008517
Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review
Abstract
Background: Variability in interferon-gamma release assays (IGRAs) results for tuberculosis has implications for interpretation of results close to the cut-point, and for defining thresholds for test conversion and reversion. However, little is known about the within-subject variability (reproducibility) of IGRAs. Several national guidelines recommend a two-step testing procedure (tuberculin skin test [TST] followed by IGRA) for the diagnosis of LTBI. However, the effect of a preceding TST on subsequent IGRA results has been reported in studies with apparently conflicting results.
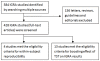
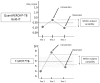
Methodology/findings: We conducted a systematic review to synthesize evidence on within-subject variability of IGRA results and the potential boosting effect of TST. We searched several databases and reviewed citations of previous reviews on IGRAs. We included studies using commercial IGRAs, in addition to non-commercial versions of the ELISPOT assay. Four studies, fulfilling our predefined criteria, examined within-subject variability and 13 studies evaluated TST effects on subsequent IGRA responses. Meta-analysis was not considered appropriate because of heterogeneity in study methods, assays, and populations. Although based on limited data, within-subject variability was present in all studies but the magnitude varied (16-80%) across studies. A TST induced "boosting" of IGRA responses was demonstrated in several studies and although more pronounced in IGRA-positive (i.e. sensitized) individuals, also occurred in a smaller but not insignificant proportion of IGRA-negative subjects. The TST appeared to affect IGRA responses only after 3 days and may apparently persist for several months, but evidence for this is weak.
Conclusions/significance: Although reproducibility data are scarce, significant within person IGRA variability has been reported. If confirmed in more studies, this has implications for the interpretation of results close to the cut-point and for definition of conversions and reversions. Although the effect of TST on IGRA results is likely to be inconsequential in IGRA-positive subjects, in IGRA-negative subjects, the interpretation of results may be confounded by a preceding TST if administered more than 3 days prior to an IGRA.
Conflict of interest statement
Figures
Similar articles
-
Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update.Ann Intern Med. 2008 Aug 5;149(3):177-84. doi: 10.7326/0003-4819-149-3-200808050-00241. Epub 2008 Jun 30. Ann Intern Med. 2008. PMID: 18593687 Free PMC article.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.Health Technol Assess. 2007 Jan;11(3):1-196. doi: 10.3310/hta11030. Health Technol Assess. 2007. PMID: 17266837
-
Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review.Thorax. 2012 Jan;67(1):62-70. doi: 10.1136/thx.2010.143180. Epub 2011 Jan 12. Thorax. 2012. PMID: 21228420
-
Comparing interferon-gamma release assays with tuberculin skin test for identifying latent tuberculosis infection that progresses to active tuberculosis: systematic review and meta-analysis.BMC Infect Dis. 2017 Mar 9;17(1):200. doi: 10.1186/s12879-017-2301-4. BMC Infect Dis. 2017. PMID: 28274215 Free PMC article.
-
Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis.Lancet Infect Dis. 2012 Jan;12(1):45-55. doi: 10.1016/S1473-3099(11)70210-9. Epub 2011 Aug 16. Lancet Infect Dis. 2012. PMID: 21846592 Free PMC article.
Cited by
-
Phenotype Definition for "Resisters" to Mycobacterium tuberculosis Infection in the Literature-A Review and Recommendations.Front Immunol. 2021 Feb 25;12:619988. doi: 10.3389/fimmu.2021.619988. eCollection 2021. Front Immunol. 2021. PMID: 33717116 Free PMC article. Review.
-
Psoriatic disease and tuberculosis nowadays.Clin Dev Immunol. 2012;2012:747204. doi: 10.1155/2012/747204. Epub 2012 May 8. Clin Dev Immunol. 2012. PMID: 22645622 Free PMC article. Review.
-
Use of interferon-gamma release assays in a health care worker screening program: experience from a tertiary care centre in the United States.Can Respir J. 2012 Mar-Apr;19(2):84-8. doi: 10.1155/2012/576324. Can Respir J. 2012. PMID: 22536576 Free PMC article.
-
Performance of the QuantiFERON®-TB Gold In-Tube assay in tuberculin skin test converters: a prospective cohort study.Int J Tuberc Lung Dis. 2018 Sep 1;22(9):1000-1006. doi: 10.5588/ijtld.18.0073. Int J Tuberc Lung Dis. 2018. PMID: 30092864 Free PMC article.
-
Evaluating Latent Tuberculosis Infection Test Performance Using Latent Class Analysis in a TB and HIV Endemic Setting.Int J Environ Res Public Health. 2019 Aug 14;16(16):2912. doi: 10.3390/ijerph16162912. Int J Environ Res Public Health. 2019. PMID: 31416206 Free PMC article.
References
-
- Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192–1204. - PubMed
-
- van Zyl-Smit RN, Pai M, Peprah K, Meldau R, Kieck J, et al. Within-subject Variability and Boosting of T Cell IFN-{gamma} Responses Following Tuberculin Skin Testing. Am J Respir Crit Care Med. 2009;180:49–58. - PubMed
-
- Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

