Explaining the imperfection of the molecular clock of hominid mitochondria
- PMID: 20041137
- PMCID: PMC2794369
- DOI: 10.1371/journal.pone.0008260
Explaining the imperfection of the molecular clock of hominid mitochondria
Abstract
The molecular clock of mitochondrial DNA has been extensively used to date various genetic events. However, its substitution rate among humans appears to be higher than rates inferred from human-chimpanzee comparisons, limiting the potential of interspecies clock calibrations for intraspecific dating. It is not well understood how and why the substitution rate accelerates. We have analyzed a phylogenetic tree of 3057 publicly available human mitochondrial DNA coding region sequences for changes in the ratios of mutations belonging to different functional classes. The proportion of non-synonymous and RNA genes substitutions has reduced over hundreds of thousands of years. The highest mutation ratios corresponding to fast acceleration in the apparent substitution rate of the coding sequence have occurred after the end of the Last Ice Age. We recalibrate the molecular clock of human mtDNA as 7990 years per synonymous mutation over the mitochondrial genome. However, the distribution of substitutions at synonymous sites in human data significantly departs from a model assuming a single rate parameter and implies at least 3 different subclasses of sites. Neutral model with 3 synonymous substitution rates can explain most, if not all, of the apparent molecular clock difference between the intra- and interspecies levels. Our findings imply the sluggishness of purifying selection in removing the slightly deleterious mutations from the human as well as the Neandertal and chimpanzee populations. However, for humans, the weakness of purifying selection has been further exacerbated by the population expansions associated with the out-of Africa migration and the end of the Last Ice Age.
Conflict of interest statement
Figures
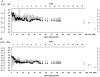
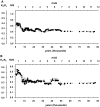

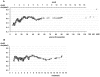
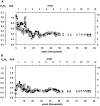
References
-
- Zuckerkandl E, Pauling L. Molecular disease, evolution, and genetic heterogeneity. In: Kasha M, Pullman B, editors. Horizons in Biochemistry. New York: Academic Press; 1962. pp. 189–225.
-
- Kumar S. Molecular clocks: four decades of evolution. Nat Rev Genet. 2005;6:654–662. - PubMed
-
- Bromham L, Penny D. The modern molecular clock. Nat Rev Genet. 2003;4:216–224. - PubMed
-
- Avise JC. Phylogeography: The History and Formation of Species. Cambridge, Massachusetts: Harvard University Press; 2000.
-
- Torroni A, Achilli A, Macaulay V, Richards M, Bandelt H. Harvesting the fruit of the human mtDNA tree. Trends Genet. 2006;22:339–345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

