Ambulatory-based standardized therapy for multi-drug resistant tuberculosis: experience from Nepal, 2005-2006
- PMID: 20041140
- PMCID: PMC2794372
- DOI: 10.1371/journal.pone.0008313
Ambulatory-based standardized therapy for multi-drug resistant tuberculosis: experience from Nepal, 2005-2006
Abstract
Objective: The aim of this study was to describe treatment outcomes for multi-drug resistant tuberculosis (MDR-TB) outpatients on a standardized regimen in Nepal.
Methodology: Data on pulmonary MDR-TB patients enrolled for treatment in the Green Light Committee-approved National Programme between 15 September 2005 and 15 September 2006 were studied. Standardized regimen was used (8Z-Km-Ofx-Eto-Cs/16Z-Ofx-Eto-Cs) for a maximum of 32 months and follow-up was by smear and culture. Drug susceptibility testing (DST) results were not used to modify the treatment regimen. MDR-TB therapy was delivered in outpatient facilities for the whole course of treatment. Multivariable analysis was used to explain bacteriological cure as a function of sex, age, initial body weight, history of previous treatment and the region of report.
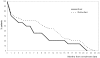
Principal findings: In the first 12-months, 175 laboratory-confirmed MDR-TB cases (62% males) had outcomes reported. Most cases had failed a Category 2 first-line regimen (87%) or a Category 1 regimen (6%), 2% were previously untreated contacts of MDR-TB cases and 5% were unspecified. Cure was reported among 70% of patients (range 38%-93% by Region), 8% died, 5% failed treatment, and 17% defaulted. Unfavorable outcomes were not correlated to the number of resistant drugs at baseline DST. Cases who died had a lower mean body weight than those surviving (40.3 kg vs 47.2 kg, p<0.05). Default was significantly higher in two regions [Eastern OR = 6.2; 95%CL2.0-18.9; Far West OR = 5.0; 95%CL1.0-24.3]. At logistic regression, cure was inversely associated with body weight <36 kg [Adj.OR = 0.1; 95%CL0.0-0.3; ref. 55-75 kg] and treatment in the Eastern region [Adj.OR = 0.1; 95%CL0.0-0.4; ref. Central region].
Conclusions: The implementation of an ambulatory-based treatment programme for MDR-TB based on a fully standardized regimen can yield high cure rates even in resource-limited settings. The determinants of unfavorable outcome should be investigated thoroughly to maximize likelihood of successful treatment.
Conflict of interest statement
Figures

Similar articles
-
Revised Category II regimen as an alternative strategy for retreatment of Category I regimen failure and irregular treatment cases.Am J Ther. 2011 Sep;18(5):343-9. doi: 10.1097/MJT.0b013e3181dd60ec. Am J Ther. 2011. PMID: 20535008
-
Ambulatory multi-drug resistant tuberculosis treatment outcomes in a cohort of HIV-infected patients in a slum setting in Mumbai, India.PLoS One. 2011;6(12):e28066. doi: 10.1371/journal.pone.0028066. Epub 2011 Dec 1. PLoS One. 2011. PMID: 22145022 Free PMC article.
-
Development of a Patient-Centred, Psychosocial Support Intervention for Multi-Drug-Resistant Tuberculosis (MDR-TB) Care in Nepal.PLoS One. 2017 Jan 18;12(1):e0167559. doi: 10.1371/journal.pone.0167559. eCollection 2017. PLoS One. 2017. PMID: 28099475 Free PMC article.
-
Multi-drug resistant tuberculosis burden and risk factors: an update.Kathmandu Univ Med J (KUMJ). 2010 Jan-Mar;8(29):116-25. doi: 10.3126/kumj.v8i1.3234. Kathmandu Univ Med J (KUMJ). 2010. PMID: 21209520 Review.
-
Delamanid: From discovery to its use for pulmonary multidrug-resistant tuberculosis (MDR-TB).Tuberculosis (Edinb). 2018 Jul;111:20-30. doi: 10.1016/j.tube.2018.04.008. Epub 2018 May 3. Tuberculosis (Edinb). 2018. PMID: 30029909 Review.
Cited by
-
Molecular characterization of multidrug-resistant Mycobacterium tuberculosis isolated in Nepal.Antimicrob Agents Chemother. 2012 Jun;56(6):2831-6. doi: 10.1128/AAC.06418-11. Epub 2012 Mar 26. Antimicrob Agents Chemother. 2012. PMID: 22450970 Free PMC article.
-
Treatment Outcomes and Adverse Drug Effects of Ethambutol, Cycloserine, and Terizidone for the Treatment of Multidrug-Resistant Tuberculosis in South Africa.Antimicrob Agents Chemother. 2020 Dec 16;65(1):e00744-20. doi: 10.1128/AAC.00744-20. Print 2020 Dec 16. Antimicrob Agents Chemother. 2020. PMID: 33046491 Free PMC article.
-
Management of multidrug-resistant TB: novel treatments and their expansion to low resource settings.Trans R Soc Trop Med Hyg. 2016 Mar;110(3):163-72. doi: 10.1093/trstmh/trv107. Trans R Soc Trop Med Hyg. 2016. PMID: 26884496 Free PMC article. Review.
-
Treatment outcomes from community-based drug resistant tuberculosis treatment programs: a systematic review and meta-analysis.BMC Infect Dis. 2014 Jun 17;14:333. doi: 10.1186/1471-2334-14-333. BMC Infect Dis. 2014. PMID: 24938738 Free PMC article.
-
Outcome of standardized treatment for patients with MDR-TB from Tamil Nadu, India.Indian J Med Res. 2011 May;133(5):529-34. Indian J Med Res. 2011. PMID: 21623039 Free PMC article. Clinical Trial.
References
-
- Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, et al. Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373(9678):1861–73. - PubMed
-
- Raviglione MC, Smith IM. XDR tuberculosis–implications for global public health. N Engl J Med. 2007;356(7):656–9. - PubMed
-
- World Health Organization. Guidelines for the programmatic management of drug resistant tuberculosis. 2008. Emergency Update (WHO/HTM/TB/2008.402). Geneva, Switzerland. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

