The ZEB1 transcription factor is a novel repressor of adiposity in female mice
- PMID: 20041147
- PMCID: PMC2794530
- DOI: 10.1371/journal.pone.0008460
The ZEB1 transcription factor is a novel repressor of adiposity in female mice
Abstract
Background: Four genome-wide association studies mapped an "obesity" gene to human chromosome 10p11-12. As the zinc finger E-box binding homeobox 1 (ZEB1) transcription factor is encoded by the TCF8 gene located in that region, and as it influences the differentiation of various mesodermal lineages, we hypothesized that ZEB1 might also modulate adiposity. The goal of these studies was to test that hypothesis in mice.
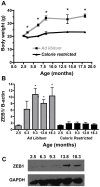
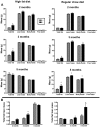
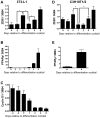
Methodology/principal findings: To ascertain whether fat accumulation affects ZEB1 expression, female C57BL/6 mice were fed a regular chow diet (RCD) ad libitum or a 25% calorie-restricted diet from 2.5 to 18.3 months of age. ZEB1 mRNA levels in parametrial fat were six to ten times higher in the obese mice. To determine directly whether ZEB1 affects adiposity, wild type (WT) mice and mice heterozygous for TCF8 (TCF8+/-) were fed an RCD or a high-fat diet (HFD) (60% calories from fat). By two months of age on an HFD and three months on an RCD, TCF8+/- mice were heavier than WT controls, which was attributed by Echo MRI to increased fat mass (at three months on an HFD: 0.517+/-0.081 total fat/lean mass versus 0.313+/-0.036; at three months on an RCD: 0.175+/-0.013 versus 0.124+/-0.012). No differences were observed in food uptake or physical activity, suggesting that the genotypes differ in some aspect of their metabolic activity. ZEB1 expression also increases during adipogenesis in cell culture.
Conclusion/significance: These results show for the first time that the ZEB1 transcription factor regulates the accumulation of adipose tissue. Furthermore, they corroborate the genome-wide association studies that mapped an "obesity" gene at chromosome 10p11-12.
Conflict of interest statement
Figures

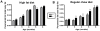


References
-
- Sabin MA, Shield JP. Childhood obesity. Front Horm Res. 2008;36:85–96. - PubMed
-
- Sekiya I, Larson BL, Vuoristo JT, Cui JG, Prockop DJ. Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J Bone Miner Res. 2004;19:256–64. - PubMed
-
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–56. - PubMed
-
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

