The role of scavenger receptor B1 in infection with Mycobacterium tuberculosis in a murine model
- PMID: 20041149
- PMCID: PMC2794535
- DOI: 10.1371/journal.pone.0008448
The role of scavenger receptor B1 in infection with Mycobacterium tuberculosis in a murine model
Abstract
Background: The interaction between Mycobacterium tuberculosis (Mtb) and host cells is complex and far from being understood. The role of the different receptor(s) implicated in the recognition of Mtb in particular remains poorly defined, and those that have been found to have activity in vitro were subsequently shown to be redundant in vivo.
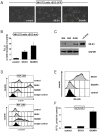
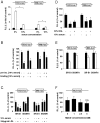
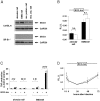
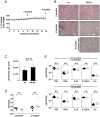
Methods and findings: To identify novel receptors involved in the recognition of Mtb, we screened a macrophage cDNA library and identified scavenger receptor B class 1 (SR-B1) as a receptor for mycobacteria. SR-B1 has been well-described as a lipoprotein receptor which mediates both the selective uptake of cholesteryl esters and the efflux of cholesterol, and has also recently been implicated in the recognition of other pathogens. We show here that mycobacteria can bind directly to SR-B1 on transfected cells, and that this interaction could be inhibited in the presence of a specific antibody to SR-B1, serum or LDL. We define a variety of macrophage populations, including alveolar macrophages, that express this receptor, however, no differences in the recognition and response to mycobacteria were observed in macrophages isolated from SR-B1(-/-) or wild type mice in vitro. Moreover, when wild type and SR-B1(-/-) animals were infected with a low dose of Mtb (100 CFU/mouse) there were no alterations in survival, bacterial burdens, granuloma formation or cytokine production in the lung. However, significant reduction in the production of TNF, IFNgamma, and IL10 were observed in SR-B1(-/-) mice following infection with a high dose of Mtb (1000 CFU/mouse), which marginally affected the size of inflammatory foci but did not influence bacterial burdens. Deficiency of SR-B1 also had no effect on resistance to disease under conditions of varying dietary cholesterol. We did observe, however, that the presence of high levels of cholesterol in the diet significantly enhanced the bacterial burdens in the lung, but this was independent of SR-B1.
Conclusion: SR-B1 is involved in mycobacterial recognition, but this receptor plays only a minor role in anti-mycobacterial immunity in vivo. Like many other receptors for these pathogens, the loss of SR-B1 can be functionally compensated for under normal conditions.
Conflict of interest statement
Figures
Similar articles
-
Identification of scavenger receptor B1 as the airway microfold cell receptor for Mycobacterium tuberculosis.Elife. 2020 Mar 5;9:e52551. doi: 10.7554/eLife.52551. Elife. 2020. PMID: 32134383 Free PMC article.
-
Scavenger receptor class B, type 1 facilitates cellular fatty acid uptake.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Feb;1865(2):158554. doi: 10.1016/j.bbalip.2019.158554. Epub 2019 Oct 31. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31678516 Free PMC article.
-
Targeted invalidation of SR-B1 in macrophages reduces macrophage apoptosis and accelerates atherosclerosis.Cardiovasc Res. 2020 Mar 1;116(3):554-565. doi: 10.1093/cvr/cvz138. Cardiovasc Res. 2020. PMID: 31119270
-
The impact of Mycobacterium tuberculosis on the macrophage cholesterol metabolism pathway.Front Immunol. 2024 May 30;15:1402024. doi: 10.3389/fimmu.2024.1402024. eCollection 2024. Front Immunol. 2024. PMID: 38873598 Free PMC article. Review.
-
Macrophage SR-B1 in atherosclerotic cardiovascular disease.Curr Opin Lipidol. 2022 Jun 1;33(3):167-174. doi: 10.1097/MOL.0000000000000822. Epub 2022 Mar 7. Curr Opin Lipidol. 2022. PMID: 35258032 Review.
Cited by
-
Gene expression profile analysis and target gene discovery of Mycobacterium tuberculosis biofilm.Appl Microbiol Biotechnol. 2021 Jun;105(12):5123-5134. doi: 10.1007/s00253-021-11361-4. Epub 2021 Jun 14. Appl Microbiol Biotechnol. 2021. PMID: 34125278
-
Changes in the Immune Phenotype and Gene Expression Profile Driven by a Novel Tuberculosis Nanovaccine: Short and Long-Term Post-immunization.Front Immunol. 2021 Jan 28;11:589863. doi: 10.3389/fimmu.2020.589863. eCollection 2020. Front Immunol. 2021. PMID: 33584654 Free PMC article.
-
Roles of cysteine in the structure and metabolic function of Mycobacterium tuberculosis CYP142A1.RSC Adv. 2022 Aug 30;12(38):24447-24455. doi: 10.1039/d2ra04257f. eCollection 2022 Aug 30. RSC Adv. 2022. PMID: 36128375 Free PMC article.
-
Lipid-Centric Design of Plasma Membrane-Mimicking Nanocarriers for Targeted Chemotherapeutic Delivery.Adv Mater. 2024 Jan;36(2):e2306808. doi: 10.1002/adma.202306808. Epub 2023 Nov 23. Adv Mater. 2024. PMID: 37732588 Free PMC article.
-
Underwhelming or Misunderstood? Genetic Variability of Pattern Recognition Receptors in Immune Responses and Resistance to Mycobacterium tuberculosis.Front Immunol. 2021 Jun 30;12:714808. doi: 10.3389/fimmu.2021.714808. eCollection 2021. Front Immunol. 2021. PMID: 34276708 Free PMC article. Review.
References
-
- Zimmerli S, Edwards S, Ernst JD. Selective receptor blockade during phagocytosis does not alter the survival and growth of Mycobacterium tuberculosis in human macrophages. Am J Respir Cell Mol Biol. 1996;15:760–770. - PubMed
-
- Wieland CW, Koppel EA, den Dunnen J, Florquin S, McKenzie AN, et al. Mice lacking SIGNR1 have stronger T helper 1 responses to Mycobacterium tuberculosis. Microbes Infect. 2007;9:134–141. - PubMed
-
- Hu C, Mayadas-Norton T, Tanaka K, Chan J, Salgame P. Mycobacterium tuberculosis infection in complement receptor 3-deficient mice. J Immunol. 2000;165:2596–2602. - PubMed
-
- Holscher C, Reiling N, Schaible UE, Holscher A, Bathmann C, et al. Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, -4 and -9. Eur J Immunol. 2008;38:680–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

