MEF2C silencing attenuates load-induced left ventricular hypertrophy by modulating mTOR/S6K pathway in mice
- PMID: 20041152
- PMCID: PMC2794538
- DOI: 10.1371/journal.pone.0008472
MEF2C silencing attenuates load-induced left ventricular hypertrophy by modulating mTOR/S6K pathway in mice
Erratum in
- PLoS One. 2014;9(10):e112344
Abstract
Background: The activation of the members of the myocyte enhancer factor-2 family (MEF2A, B, C and D) of transcription factors promotes cardiac hypertrophy and failure. However, the role of its individual components in the pathogenesis of cardiac hypertrophy remains unclear.
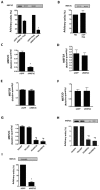
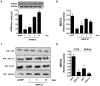
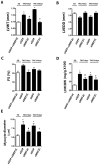
Methodology/principal findings: In this study, we investigated whether MEF2C plays a role in mediating the left ventricular hypertrophy by pressure overload in mice. The knockdown of myocardial MEF2C induced by specific small interfering RNA (siRNA) has been shown to attenuate hypertrophy, interstitial fibrosis and the rise of ANP levels in aortic banded mice. We detected that the depletion of MEF2C also results in lowered levels of both PGC-1alpha and mitochondrial DNA in the overloaded left ventricle, associated with enhanced AMP:ATP ratio. Additionally, MEF2C depletion was accompanied by defective activation of S6K in response to pressure overload. Treatment with the amino acid leucine stimulated S6K and suppressed the attenuation of left ventricular hypertrophy and fibrosis in the aforementioned aortic banded mice.
Conclusion/significance: These findings represent new evidences that MEF2C depletion attenuates the hypertrophic responses to mechanical stress and highlight the potential of MEF2C to be a target for new therapies to cardiac hypertrophy and failure.
Conflict of interest statement
Figures




References
-
- Katz AM. The cardiomyopathy of overload: an unnatural growth response in the hypertrophied heart. Ann Intern Med. 1994;121:363–371. - PubMed
-
- Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. - PubMed
-
- Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141:334–341. - PubMed
-
- Diwan A, Dorn GW., 2nd Decompensation of cardiac hypertrophy: cellular mechanisms and novel therapeutic targets. Physiology (Bethesda) 2007;22:56–64. - PubMed
-
- Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–558. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

