A novel phase variation mechanism in the meningococcus driven by a ligand-responsive repressor and differential spacing of distal promoter elements
- PMID: 20041170
- PMCID: PMC2791445
- DOI: 10.1371/journal.ppat.1000710
A novel phase variation mechanism in the meningococcus driven by a ligand-responsive repressor and differential spacing of distal promoter elements
Abstract
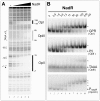
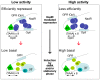
Phase variable expression, mediated by high frequency reversible changes in the length of simple sequence repeats, facilitates adaptation of bacterial populations to changing environments and is frequently important in bacterial virulence. Here we elucidate a novel phase variable mechanism for NadA, an adhesin and invasin of Neisseria meningitidis. The NadR repressor protein binds to operators flanking the phase variable tract and contributes to the differential expression levels of phase variant promoters with different numbers of repeats likely due to different spacing between operators. We show that IHF binds between these operators, and may permit looping of the promoter, allowing interaction of NadR at operators located distally or overlapping the promoter. The 4-hydroxyphenylacetic acid, a metabolite of aromatic amino acid catabolism that is secreted in saliva, induces NadA expression by inhibiting the DNA binding activity of the repressor. When induced, only minor differences are evident between NadR-independent transcription levels of promoter phase variants and are likely due to differential RNA polymerase contacts leading to altered promoter activity. Our results suggest that NadA expression is under both stochastic and tight environmental-sensing regulatory control, both mediated by the NadR repressor, and may be induced during colonization of the oropharynx where it plays a major role in the successful adhesion and invasion of the mucosa. Hence, simple sequence repeats in promoter regions may be a strategy used by host-adapted bacterial pathogens to randomly switch between expression states that may nonetheless still be induced by appropriate niche-specific signals.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Molecular Basis of Ligand-Dependent Regulation of NadR, the Transcriptional Repressor of Meningococcal Virulence Factor NadA.PLoS Pathog. 2016 Apr 22;12(4):e1005557. doi: 10.1371/journal.ppat.1005557. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27105075 Free PMC article.
-
Structural insight into the mechanism of DNA-binding attenuation of the Neisserial adhesin repressor NadR by the small natural ligand 4-hydroxyphenylacetic acid.Biochemistry. 2012 Aug 28;51(34):6738-52. doi: 10.1021/bi300656w. Epub 2012 Aug 15. Biochemistry. 2012. PMID: 22834735
-
In the NadR regulon, adhesins and diverse meningococcal functions are regulated in response to signals in human saliva.J Bacteriol. 2012 Jan;194(2):460-74. doi: 10.1128/JB.06161-11. Epub 2011 Nov 11. J Bacteriol. 2012. PMID: 22081399 Free PMC article.
-
MtrR control of a transcriptional regulatory pathway in Neisseria meningitidis that influences expression of a gene (nadA) encoding a vaccine candidate.PLoS One. 2013;8(2):e56097. doi: 10.1371/journal.pone.0056097. Epub 2013 Feb 8. PLoS One. 2013. PMID: 23409129 Free PMC article.
-
Type-4 pili and meningococcal adhesiveness.Gene. 1997 Jun 11;192(1):149-53. doi: 10.1016/s0378-1119(96)00802-5. Gene. 1997. PMID: 9224885 Review.
Cited by
-
Transcriptional regulation of the nadA gene in Neisseria meningitidis impacts the prediction of coverage of a multicomponent meningococcal serogroup B vaccine.Infect Immun. 2013 Feb;81(2):560-9. doi: 10.1128/IAI.01085-12. Epub 2012 Dec 10. Infect Immun. 2013. PMID: 23230289 Free PMC article.
-
Molecular Basis of Ligand-Dependent Regulation of NadR, the Transcriptional Repressor of Meningococcal Virulence Factor NadA.PLoS Pathog. 2016 Apr 22;12(4):e1005557. doi: 10.1371/journal.ppat.1005557. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27105075 Free PMC article.
-
How the Knowledge of Interactions between Meningococcus and the Human Immune System Has Been Used to Prepare Effective Neisseria meningitidis Vaccines.J Immunol Res. 2015;2015:189153. doi: 10.1155/2015/189153. Epub 2015 Aug 17. J Immunol Res. 2015. PMID: 26351643 Free PMC article. Review.
-
Biological Functions of the Secretome of Neisseria meningitidis.Front Cell Infect Microbiol. 2017 Jun 16;7:256. doi: 10.3389/fcimb.2017.00256. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28670572 Free PMC article. Review.
-
Virulence Factors of Meningitis-Causing Bacteria: Enabling Brain Entry across the Blood-Brain Barrier.Int J Mol Sci. 2019 Oct 29;20(21):5393. doi: 10.3390/ijms20215393. Int J Mol Sci. 2019. PMID: 31671896 Free PMC article. Review.
References
-
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378–1388. - PubMed
-
- Tinsley C, Nassif X. Meningococcal pathogenesis: at the boundary between the pre- and post-genomic eras. Curr Opin Microbiol. 2001;4:47–52. - PubMed
-
- Capecchi B, Adu-Bobie J, Di Marcello F, Ciucchi L, Masignani V, et al. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol Microbiol. 2005;55:687–698. - PubMed
-
- Pizza M, Scarlato V, Masignani V, Giuliani MM, Arico B, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–1820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

