Increased light exposure alleviates one form of photoreceptor degeneration marked by elevated calcium in the dark
- PMID: 20041177
- PMCID: PMC2793020
- DOI: 10.1371/journal.pone.0008438
Increased light exposure alleviates one form of photoreceptor degeneration marked by elevated calcium in the dark
Abstract
Background: In one group of gene mutations that cause photoreceptor degeneration in human patients, guanylyl cyclase is overactive in the dark. The ensuing excess opening of cGMP-gated cation channels causes intracellular calcium to rise to toxic levels. The Y99C mutation in guanylate cyclase-activating protein 1 (GCAP1) has been shown to act this way. We determined whether prolonged light exposure, which lowers cGMP levels through activation of phototransduction, might protect photoreceptors in a line of transgenic mice carrying the GCAP1-Y99C.
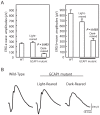
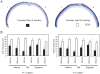
Methodology/principal findings: We reared cohorts of GCAP1-Y99C transgenic mice under standard cyclic, constant dark and constant light conditions. Mouse eyes were analyzed by histology and by immunofluorescence for GFAP upregulation, a non-specific marker for photoreceptor degeneration. Full-field electroretinograms (ERGs) were recorded to assess retinal function. Consistent with our hypothesis, constant darkness accelerated disease, while continuous lighting arrested photoreceptor degeneration.
Conclusions/significance: In contrast to most forms of retinal degeneration, which are exacerbated by increased exposure to ambient light, a subset with mutations that cause overly active guanylyl cyclase and high intracellular calcium benefitted from prolonged light exposure. These findings may have therapeutic implications for patients with these types of genetic defects.
Conflict of interest statement
Figures


Similar articles
-
The Y99C mutation in guanylyl cyclase-activating protein 1 increases intracellular Ca2+ and causes photoreceptor degeneration in transgenic mice.J Neurosci. 2004 Jul 7;24(27):6078-85. doi: 10.1523/JNEUROSCI.0963-04.2004. J Neurosci. 2004. PMID: 15240799 Free PMC article.
-
GUCY2D Cone-Rod Dystrophy-6 Is a "Phototransduction Disease" Triggered by Abnormal Calcium Feedback on Retinal Membrane Guanylyl Cyclase 1.J Neurosci. 2018 Mar 21;38(12):2990-3000. doi: 10.1523/JNEUROSCI.2985-17.2018. Epub 2018 Feb 12. J Neurosci. 2018. PMID: 29440533 Free PMC article.
-
Retinal degeneration-3 protein promotes photoreceptor survival by suppressing activation of guanylyl cyclase rather than accelerating GMP recycling.J Biol Chem. 2021 Jan-Jun;296:100362. doi: 10.1016/j.jbc.2021.100362. Epub 2021 Feb 2. J Biol Chem. 2021. PMID: 33539922 Free PMC article.
-
Factors that affect regulation of cGMP synthesis in vertebrate photoreceptors and their genetic link to human retinal degeneration.Mol Cell Biochem. 2002 Jan;230(1-2):139-47. Mol Cell Biochem. 2002. PMID: 11952089 Review.
-
Regulation of photoreceptor membrane guanylyl cyclases by guanylyl cyclase activator proteins.Methods. 1999 Dec;19(4):521-31. doi: 10.1006/meth.1999.0894. Methods. 1999. PMID: 10581151 Review.
Cited by
-
Signalling in the genomic era.J Cell Commun Signal. 2010 Oct;4(3):115-7. doi: 10.1007/s12079-010-0091-1. Epub 2010 Jun 6. J Cell Commun Signal. 2010. PMID: 21063501 Free PMC article.
-
Retinal guanylyl cyclase isozyme 1 is the preferential in vivo target for constitutively active GCAP1 mutants causing congenital degeneration of photoreceptors.J Neurosci. 2012 May 23;32(21):7208-17. doi: 10.1523/JNEUROSCI.0976-12.2012. J Neurosci. 2012. PMID: 22623665 Free PMC article.
-
Anesthetic effects on electrophysiological responses across the visual pathway.Sci Rep. 2024 Nov 13;14(1):27825. doi: 10.1038/s41598-024-79240-2. Sci Rep. 2024. PMID: 39537872 Free PMC article.
-
Long-term RNA interference gene therapy in a dominant retinitis pigmentosa mouse model.Proc Natl Acad Sci U S A. 2011 Nov 8;108(45):18476-81. doi: 10.1073/pnas.1112758108. Epub 2011 Oct 31. Proc Natl Acad Sci U S A. 2011. PMID: 22042849 Free PMC article.
-
Cyclic nucleotide-gated channel block by hydrolysis-resistant tetracaine derivatives.J Med Chem. 2011 Jul 14;54(13):4904-12. doi: 10.1021/jm200495g. Epub 2011 Jun 14. J Med Chem. 2011. PMID: 21634421 Free PMC article.
References
-
- Berson EL. Light deprivation for early retinitis pigmentosa. A hypothesis. Arch Ophthalmol. 1971;85:521–529. - PubMed
-
- LaVail MM, Battelle BA. Influence of eye pigmentation and light deprivation on inherited retinal dystrophy in the rat. Exp Eye Res. 1975;21:167–192. - PubMed
-
- Heckenlively JR, Rodriguez JA, Daiger SP. Autosomal dominant sectoral retinitis pigmentosa. Two families with transversion mutation in codon 23 of rhodopsin. Arch Ophthalmol. 1991;109:84–91. - PubMed
-
- Naash ML, Peachey NS, Li ZY, Gryczan CC, Goto Y, et al. Light-induced acceleration of photoreceptor degeneration in transgenic mice expressing mutant rhodopsin. Invest Ophthalmol Vis Sci. 1996;37:775–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

