Fiber mediated receptor masking in non-infected bystander cells restricts adenovirus cell killing effect but promotes adenovirus host co-existence
- PMID: 20041185
- PMCID: PMC2793518
- DOI: 10.1371/journal.pone.0008484
Fiber mediated receptor masking in non-infected bystander cells restricts adenovirus cell killing effect but promotes adenovirus host co-existence
Abstract
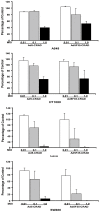
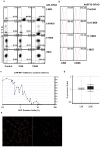
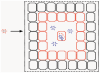
The basic concept of conditionally replicating adenoviruses (CRAD) as oncolytic agents is that progenies generated from each round of infection will disperse, infect and kill new cancer cells. However, CRAD has only inhibited, but not eradicated tumor growth in xenograft tumor therapy, and CRAD therapy has had only marginal clinical benefit to cancer patients. Here, we found that CRAD propagation and cancer cell survival co-existed for long periods of time when infection was initiated at low multiplicity of infection (MOI), and cancer cell killing was inefficient and slow compared to the assumed cell killing effect upon infection at high MOI. Excessive production of fiber molecules from initial CRAD infection of only 1 to 2% cancer cells and their release prior to the viral particle itself caused a tropism-specific receptor masking in both infected and non-infected bystander cells. Consequently, the non-infected bystander cells were inefficiently bound and infected by CRAD progenies. Further, fiber overproduction with concomitant restriction of adenovirus spread was observed in xenograft cancer therapy models. Besides the CAR-binding Ad4, Ad5, and Ad37, infection with CD46-binding Ad35 and Ad11 also caused receptor masking. Fiber overproduction and its resulting receptor masking thus play a key role in limiting CRAD functionality, but potentially promote adenovirus and host cell co-existence. These findings also give important clues for understanding mechanisms underlying the natural infection course of various adenoviruses.
Conflict of interest statement
Figures





Similar articles
-
Efficient antitumor effects of carrier cells loaded with a fiber-substituted conditionally replicating adenovirus on CAR-negative tumor cells.Cancer Gene Ther. 2012 Feb;19(2):118-25. doi: 10.1038/cgt.2011.74. Epub 2011 Nov 11. Cancer Gene Ther. 2012. PMID: 22076042
-
5/35 fiber-modified conditionally replicative adenovirus armed with p53 shows increased tumor-suppressing capacity to breast cancer cells.Hum Gene Ther. 2011 Mar;22(3):283-92. doi: 10.1089/hum.2010.058. Epub 2011 Jan 11. Hum Gene Ther. 2011. PMID: 20846024
-
A capsid-modified, conditionally replicating oncolytic adenovirus vector expressing TRAIL Leads to enhanced cancer cell killing in human glioblastoma models.Cancer Res. 2007 Sep 15;67(18):8783-90. doi: 10.1158/0008-5472.CAN-07-0357. Cancer Res. 2007. PMID: 17875719
-
A novel fiber chimeric conditionally replicative adenovirus-Ad5/F35 for tumor therapy.Cancer Biol Ther. 2017 Nov 2;18(11):833-840. doi: 10.1080/15384047.2017.1395115. Epub 2017 Nov 16. Cancer Biol Ther. 2017. PMID: 29144842 Free PMC article. Review.
-
Adenoviruses types, cell receptors and local innate cytokines in adenovirus infection.Int Rev Immunol. 2014 Jan;33(1):45-53. doi: 10.3109/08830185.2013.823420. Epub 2013 Oct 15. Int Rev Immunol. 2014. PMID: 24127823 Review.
Cited by
-
Viral capsid is a pathogen-associated molecular pattern in adenovirus keratitis.PLoS Pathog. 2010 Apr 15;6(4):e1000841. doi: 10.1371/journal.ppat.1000841. PLoS Pathog. 2010. PMID: 20419141 Free PMC article.
-
Modeling adenovirus latency in human lymphocyte cell lines.J Virol. 2010 Sep;84(17):8799-810. doi: 10.1128/JVI.00562-10. Epub 2010 Jun 23. J Virol. 2010. PMID: 20573817 Free PMC article.
-
Targeting adenoviruses with factor x-single-chain antibody fusion proteins.Hum Gene Ther. 2010 Jun;21(6):739-49. doi: 10.1089/hum.2009.190. Hum Gene Ther. 2010. PMID: 20331369 Free PMC article.
-
Oncolytic Adenoviruses in Cancer Treatment.Biomedicines. 2014 Feb 21;2(1):36-49. doi: 10.3390/biomedicines2010036. Biomedicines. 2014. PMID: 28548059 Free PMC article. Review.
-
Adenovirus-expressed preS2 antibody inhibits hepatitis B virus infection and hepatic carcinogenesis.World J Gastroenterol. 2012 Jan 28;18(4):349-55. doi: 10.3748/wjg.v18.i4.349. World J Gastroenterol. 2012. PMID: 22294841 Free PMC article.
References
-
- Fox JP, Hall CE, Cooney MK. The Seattle Virus Watch. VII. Observations of adenovirus infections. Am J Epidemiol. 1977;105(4):362–386. - PubMed
-
- Lichtenstein DL, Wold WS. Experimental infections of humans with wild-type adenoviruses and with replication-competent adenovirus vectors: replication, safety, and transmission. Cancer Gene Ther. 2004;11(12):819–829. - PubMed
-
- Edqvist A, Rebetz J, Jaras M, Rydelius A, Skagerberg G, et al. Detection of cell cycle- and differentiation stage-dependent human telomerase reverse transcriptase expression in single living cancer cells. Mol Ther. 2006;14(1):139–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

