CX3CR1 is expressed by human B lymphocytes and mediates [corrected] CX3CL1 driven chemotaxis of tonsil centrocytes
- PMID: 20041188
- PMCID: PMC2793522
- DOI: 10.1371/journal.pone.0008485
CX3CR1 is expressed by human B lymphocytes and mediates [corrected] CX3CL1 driven chemotaxis of tonsil centrocytes
Erratum in
- PLoS One. 2010;5(1). doi: 10.1371/annotation/50da3cf8-38cb-4985-8875-e41aa03191ba
Abstract
Background: Fractalkine/CX(3)CL1, a surface chemokine, binds to CX(3)CR1 expressed by different lymphocyte subsets. Since CX(3)CL1 has been detected in the germinal centres of secondary lymphoid tissue, in this study we have investigated CX(3)CR1 expression and function in human naïve, germinal centre and memory B cells isolated from tonsil or peripheral blood.
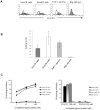
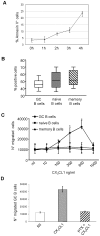
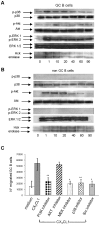
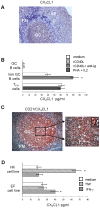
Methodology/principal findings: We demonstrate unambiguously that highly purified human B cells from tonsil and peripheral blood expressed CX(3)CR1 at mRNA and protein levels as assessed by quantitative PCR, flow cytometry and competition binding assays. In particular, naïve, germinal centre and memory B cells expressed CX(3)CR1 but only germinal centre B cells were attracted by soluble CX(3)CL1 in a transwell assay. CX(3)CL1 signalling in germinal centre B cells involved PI3K, Erk1/2, p38, and Src phosphorylation, as assessed by Western blot experiments. CX(3)CR1(+) germinal centre B cells were devoid of centroblasts and enriched for centrocytes that migrated to soluble CX(3)CL1. ELISA assay showed that soluble CX(3)CL1 was secreted constitutively by follicular dendritic cells and T follicular helper cells, two cell populations homing in the germinal centre light zone as centrocytes. At variance with that observed in humans, soluble CX(3)CL1 did not attract spleen B cells from wild type mice. OVA immunized CX(3)CR1(-/-) or CX(3)CL1(-/-) mice showed significantly decreased specific IgG production compared to wild type mice.
Conclusion/significance: We propose a model whereby human follicular dendritic cells and T follicular helper cells release in the light zone of germinal centre soluble CX(3)CL1 that attracts centrocytes. The functional implications of these results warrant further investigation.
Conflict of interest statement
Figures



Similar articles
-
CX3CL1/fractalkine is a novel regulator of normal and malignant human B cell function.J Leukoc Biol. 2012 Jul;92(1):51-8. doi: 10.1189/jlb.0112035. Epub 2012 Mar 27. J Leukoc Biol. 2012. PMID: 22457367 Review.
-
A novel role of the CX3CR1/CX3CL1 system in the cross-talk between chronic lymphocytic leukemia cells and tumor microenvironment.Leukemia. 2011 Aug;25(8):1268-77. doi: 10.1038/leu.2011.88. Epub 2011 May 6. Leukemia. 2011. PMID: 21546901
-
Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge.Brain Behav Immun. 2010 Oct;24(7):1190-201. doi: 10.1016/j.bbi.2010.05.011. Epub 2010 Jun 4. Brain Behav Immun. 2010. PMID: 20570721 Free PMC article.
-
The role of CX₃CL1/CX₃CR1 in pulmonary angiogenesis and intravascular monocyte accumulation in rat experimental hepatopulmonary syndrome.J Hepatol. 2012 Oct;57(4):752-8. doi: 10.1016/j.jhep.2012.05.014. Epub 2012 May 29. J Hepatol. 2012. PMID: 22659346 Free PMC article.
-
Selective Dependence of Kidney Dendritic Cells on CX3CR1--Implications for Glomerulonephritis Therapy.Adv Exp Med Biol. 2015;850:55-71. doi: 10.1007/978-3-319-15774-0_5. Adv Exp Med Biol. 2015. PMID: 26324346 Review.
Cited by
-
CX3CR1(+) B cells show immune suppressor properties.J Biol Chem. 2014 Aug 15;289(33):22630-22635. doi: 10.1074/jbc.M114.569459. Epub 2014 Jun 26. J Biol Chem. 2014. PMID: 24970890 Free PMC article. Clinical Trial.
-
IL-25 dampens the growth of human germinal center-derived B-cell non Hodgkin Lymphoma by curtailing neoangiogenesis.Oncoimmunology. 2017 Nov 27;7(3):e1397249. doi: 10.1080/2162402X.2017.1397249. eCollection 2018. Oncoimmunology. 2017. PMID: 29399397 Free PMC article.
-
Role of fractalkine/CX3CL1 and its receptor in the pathogenesis of inflammatory and malignant diseases with emphasis on B cell malignancies.Mediators Inflamm. 2014;2014:480941. doi: 10.1155/2014/480941. Epub 2014 Mar 30. Mediators Inflamm. 2014. PMID: 24799766 Free PMC article. Review.
-
Fractalkine receptor polymorphism and chronic tonsillitis.Eur Arch Otorhinolaryngol. 2014 Jul;271(7):2045-8. doi: 10.1007/s00405-014-2908-7. Epub 2014 Feb 5. Eur Arch Otorhinolaryngol. 2014. PMID: 24496565
-
Molecular analysis of lymphoid tissue from rhesus macaque rhadinovirus-infected monkeys identifies alterations in host genes associated with oncogenesis.PLoS One. 2020 Feb 4;15(2):e0228484. doi: 10.1371/journal.pone.0228484. eCollection 2020. PLoS One. 2020. PMID: 32017809 Free PMC article.
References
-
- Umehara H, Bloom E, Okazaki T, Domae N, Imai T. Fractalkine and vascular injury. Trends Immunol. 2001;22:602–607. - PubMed
-
- Foussat A, Bouchet-Delbos L, Berrebi D, Durand-Gasselin I, Coulomb-L'Hermine A, et al. Deregulation of the expression of the fractalkine/fractalkine receptor complex in HIV-1-infected patients. Blood. 2001;98:1678–1686. - PubMed
-
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. - PubMed
-
- Imaizumi T, Yoshida H, Satoh K. Regulation of CX3CL1/fractalkine expression in endothelial cells. J Atheroscler Thromb. 2004;11:15–21. - PubMed
-
- Papadopoulos EJ, Fitzhugh DJ, Tkaczyk C, Gilfillan AM, Sassetti C, et al. Mast cells migrate, but do not degranulate, in response to fractalkine, a membrane-bound chemokine expressed constitutively in diverse cells of the skin. Eur J Immunol. 2000;30:2355–2361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

