Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice
- PMID: 20041189
- PMCID: PMC2793523
- DOI: 10.1371/journal.pone.0008474
Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice
Abstract
Background: Lupus nephritis is characterized by deposition of chromatin fragment-IgG complexes in the mesangial matrix and glomerular basement membranes (GBM). The latter defines end-stage disease. METHODOLOGY/PRINCIPALS: In the present study we determined the impact of antibodies to dsDNA, renal Dnase1 and matrix metalloprotease (MMP) mRNA levels and enzyme activities on early and late events in murine lupus nephritis. The major focus was to analyse if these factors were interrelated, and if changes in their expression explain basic processes accounting for lupus nephritis.
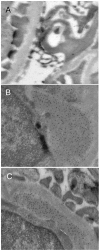
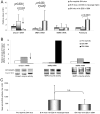
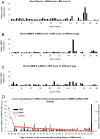
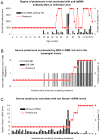
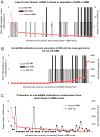
Findings: Early phases of nephritis were associated with chromatin-IgG complex deposition in the mesangial matrix. A striking observation was that this event correlated with appearance of anti-dsDNA antibodies and mild or clinically silent nephritis. These events preceded down-regulation of renal Dnase1. Later, renal Dnase1 mRNA level and enzyme activity were reduced, while MMP2 mRNA level and enzyme activity increased. Reduced levels of renal Dnase1 were associated in time with deficient fragmentation of chromatin from dead cells. Large fragments were retained and accumulated in GBM. Also, since chromatin fragments are prone to stimulate Toll-like receptors in e.g. dendritic cells, this may in fact explain increased expression of MMPs.
Significance: These scenarios may explain the basis for deposition of chromatin-IgG complexes in glomeruli in early and late stages of nephritis, loss of glomerular integrity and finally renal failure.
Conflict of interest statement
Figures
References
-
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. - PubMed
-
- Qureshi F, Yang Y, Jaques SM, Johnson MP, Naparstek Y, et al. Anti-DNA antibodies cross-reacting with laminin inhibit trophoblast attachment and migration: implications for recurrent pregnancy loss in SLE patients. Am J Reprod Immunol. 2000;44:136–142. - PubMed
-
- Sabbaga J, Line SR, Potocnjak P, Madaio MP. A murine nephritogenic monoclonal anti-DNA autoantibody binds directly to mouse laminin, the major non-collagenous protein component of the glomerular basement membrane. Eur J Immunol. 1989;19:137–143. - PubMed
-
- Amital H, Heilweil M, Ulmansky R, Szafer F, Bar-Tana R, et al. Treatment with a laminin-derived peptide suppresses lupus nephritis. J Immunol. 2005;175:5516–5523. - PubMed
-
- Mostoslavsky G, Fischel R, Yachimovich N, Yarkoni Y, Rosenmann E, et al. Lupus anti-DNA autoantibodies cross-react with a glomerular structural protein: a case for tissue injury by molecular mimicry. Eur J Immunol. 2001;31:1221–1227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

