Chylomicrons promote intestinal absorption and systemic dissemination of dietary antigen (ovalbumin) in mice
- PMID: 20041190
- PMCID: PMC2793525
- DOI: 10.1371/journal.pone.0008442
Chylomicrons promote intestinal absorption and systemic dissemination of dietary antigen (ovalbumin) in mice
Abstract
Background: A small fraction of dietary protein survives enzymatic degradation and is absorbed in potentially antigenic form. This can trigger inflammatory responses in patients with celiac disease or food allergies, but typically induces systemic immunological tolerance (oral tolerance). At present it is not clear how dietary antigens are absorbed. Most food staples, including those with common antigens such as peanuts, eggs, and milk, contain long-chain triglycerides (LCT), which stimulate mesenteric lymph flux and postprandial transport of chylomicrons through mesenteric lymph nodes (MLN) and blood. Most dietary antigens, like ovalbumin (OVA), are emulsifiers, predicting affinity for chylomicrons. We hypothesized that chylomicron formation promotes intestinal absorption and systemic dissemination of dietary antigens.
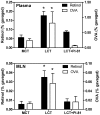
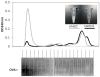
Methodology/principal findings: Absorption of OVA into MLN and blood was significantly enhanced when OVA was gavaged into fasted mice together with LCT compared with medium-chain triglycerides (MCT), which do not stimulate chylomicron formation. The effect of LCT was blocked by the addition of an inhibitor of chylomicron secretion, Pluronic L-81. Adoptively transferred OVA-specific DO11.10 T-cells proliferated more extensively in peripheral lymph nodes when OVA was gavaged with LCT than with MCT or LCT plus Pluronic L-81, suggesting that dietary OVA is systemically disseminated. Most dietary OVA in plasma was associated with chylomicrons, suggesting that these particles mediate systemic antigen dissemination. Intestinal-epithelial CaCo-2 cells secreted more cell-associated, exogenous OVA when stimulated with oleic-acid than with butyric acid, and the secreted OVA appeared to be associated with chylomicrons.
Conclusions/significance: Postprandial chylomicron formation profoundly affects absorption and systemic dissemination of dietary antigens. The fat content of a meal may affect immune responses to dietary antigens by modulating antigen absorption and transport.
Conflict of interest statement
Figures



Similar articles
-
T-lymphocyte responses to intestinally absorbed antigens can contribute to adipose tissue inflammation and glucose intolerance during high fat feeding.PLoS One. 2010 Nov 11;5(11):e13951. doi: 10.1371/journal.pone.0013951. PLoS One. 2010. PMID: 21085605 Free PMC article.
-
Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice.J Allergy Clin Immunol. 2013 Feb;131(2):442-50. doi: 10.1016/j.jaci.2012.10.011. Epub 2012 Nov 22. J Allergy Clin Immunol. 2013. PMID: 23182172 Free PMC article.
-
Chylomicrons promote intestinal absorption of lipopolysaccharides.J Lipid Res. 2009 Jan;50(1):90-7. doi: 10.1194/jlr.M800156-JLR200. Epub 2008 Sep 24. J Lipid Res. 2009. PMID: 18815435
-
Macronutrient intake and modulation on chylomicron production and clearance.Atheroscler Suppl. 2008 Sep;9(2):45-8. doi: 10.1016/j.atherosclerosissup.2008.05.006. Epub 2008 Jul 1. Atheroscler Suppl. 2008. PMID: 18595783 Review.
-
Signposts in the assembly of chylomicrons.Front Biosci. 2001 Mar 1;6:D320-31. doi: 10.2741/hussain. Front Biosci. 2001. PMID: 11229873 Review.
Cited by
-
Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome.Am J Physiol Heart Circ Physiol. 2012 Feb 1;302(3):H643-53. doi: 10.1152/ajpheart.00606.2011. Epub 2011 Dec 9. Am J Physiol Heart Circ Physiol. 2012. PMID: 22159997 Free PMC article.
-
Caloric restriction chronically impairs metabolic programming in mice.Diabetes. 2012 Nov;61(11):2734-42. doi: 10.2337/db11-1621. Epub 2012 Jul 10. Diabetes. 2012. PMID: 22787140 Free PMC article.
-
Impaired B Cell Function in Mice Lacking Perforin-2.Front Immunol. 2020 Feb 27;11:328. doi: 10.3389/fimmu.2020.00328. eCollection 2020. Front Immunol. 2020. PMID: 32180773 Free PMC article.
-
High-fat-diet-associated intestinal microbiota exacerbates psoriasis-like inflammation by enhancing systemic γδ T cell IL-17 production.Cell Rep. 2023 Jul 25;42(7):112713. doi: 10.1016/j.celrep.2023.112713. Epub 2023 Jul 7. Cell Rep. 2023. PMID: 37421628 Free PMC article.
-
T-lymphocyte responses to intestinally absorbed antigens can contribute to adipose tissue inflammation and glucose intolerance during high fat feeding.PLoS One. 2010 Nov 11;5(11):e13951. doi: 10.1371/journal.pone.0013951. PLoS One. 2010. PMID: 21085605 Free PMC article.
References
-
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2(4):361–367. - PubMed
-
- Heyman M. Symposium on ‘dietary influences on mucosal immunity’. how dietary antigens access the mucosal immune system. Proc Nutr Soc. 2001;60(4):419–426. - PubMed
-
- Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: Physiological rearrangement of tight junctions. J Membr Biol. 1990;116(2):177–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

