Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa
- PMID: 20041211
- PMCID: PMC2790608
- DOI: 10.1371/journal.ppat.1000704
Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa
Abstract
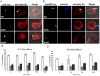
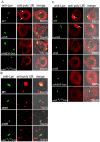
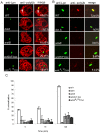
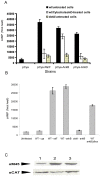
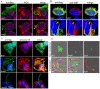
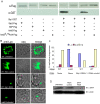
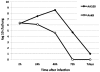
The ability of Legionella pneumophila to proliferate within various protozoa in the aquatic environment and in macrophages indicates a remarkable evolution and microbial exploitation of evolutionarily conserved eukaryotic processes. Ankyrin B (AnkB) of L. pneumophila is a non-canonical F-box-containing protein, and is the only known Dot/Icm-translocated effector of L. pneumophila essential for intra-vacuolar proliferation within both macrophages and protozoan hosts. We show that the F-box domain of AnkB and the (9)L(10)P conserved residues are essential for intracellular bacterial proliferation and for rapid acquisition of polyubiquitinated proteins by the Legionella-containing vacuole (LCV) within macrophages, Dictyostelium discoideum, and Acanthamoeba. Interestingly, translocation of AnkB and recruitment of polyubiquitinated proteins in macrophages and Acanthamoeba is rapidly triggered by extracellular bacteria within 5 min of bacterial attachment. Ectopically expressed AnkB within mammalian cells is localized to the periphery of the cell where it co-localizes with host SKP1 and recruits polyubiquitinated proteins, which results in restoration of intracellular growth to the ankB mutant similar to the parental strain. While an ectopically expressed AnkB-(9)L(10)P/AA variant is localized to the cell periphery, it does not recruit polyubiquitinated proteins and fails to trans-rescue the ankB mutant intracellular growth defect. Direct in vivo interaction of AnkB but not the AnkB-(9)L(10)P/AA variant with the host SKP1 is demonstrated. Importantly, RNAi-mediated silencing of expression of SKP1 renders the cells non-permissive for intracellular proliferation of L. pneumophila. The role of AnkB in exploitation of the polyubiquitination machinery is essential for intrapulmonary bacterial proliferation in the mouse model of Legionnaires' disease. Therefore, AnkB exhibits a novel molecular and functional mimicry of eukaryotic F-box proteins that exploits conserved polyubiquitination machinery for intracellular proliferation within evolutionarily distant hosts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Indispensable role for the eukaryotic-like ankyrin domains of the ankyrin B effector of Legionella pneumophila within macrophages and amoebae.Infect Immun. 2010 May;78(5):2079-88. doi: 10.1128/IAI.01450-09. Epub 2010 Mar 1. Infect Immun. 2010. PMID: 20194593 Free PMC article.
-
Molecular Characterization of Exploitation of the Polyubiquitination and Farnesylation Machineries of Dictyostelium Discoideum by the AnkB F-Box Effector of Legionella Pneumophila.Front Microbiol. 2011 Feb 14;2:23. doi: 10.3389/fmicb.2011.00023. eCollection 2011. Front Microbiol. 2011. PMID: 21687415 Free PMC article.
-
Exploitation of conserved eukaryotic host cell farnesylation machinery by an F-box effector of Legionella pneumophila.J Exp Med. 2010 Aug 2;207(8):1713-26. doi: 10.1084/jem.20100771. Epub 2010 Jul 26. J Exp Med. 2010. PMID: 20660614 Free PMC article.
-
Cellular microbiology and molecular ecology of Legionella-amoeba interaction.Virulence. 2013 May 15;4(4):307-14. doi: 10.4161/viru.24290. Epub 2013 Mar 27. Virulence. 2013. PMID: 23535283 Free PMC article. Review.
-
Acanthamoeba and Dictyostelium as Cellular Models for Legionella Infection.Front Cell Infect Microbiol. 2018 Mar 2;8:61. doi: 10.3389/fcimb.2018.00061. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29552544 Free PMC article. Review.
Cited by
-
Cell biology of infection by Legionella pneumophila.Microbes Infect. 2013 Feb;15(2):157-67. doi: 10.1016/j.micinf.2012.11.001. Epub 2012 Nov 14. Microbes Infect. 2013. PMID: 23159466 Free PMC article. Review.
-
The professional phagocyte Dictyostelium discoideum as a model host for bacterial pathogens.Curr Drug Targets. 2011 Jun;12(7):942-54. doi: 10.2174/138945011795677782. Curr Drug Targets. 2011. PMID: 21366522 Free PMC article. Review.
-
Hell's BELs: bacterial E3 ligases that exploit the eukaryotic ubiquitin machinery.PLoS Pathog. 2014 Aug 14;10(8):e1004255. doi: 10.1371/journal.ppat.1004255. eCollection 2014 Aug. PLoS Pathog. 2014. PMID: 25121772 Free PMC article. Review. No abstract available.
-
Proteomic analysis of a mosquito host cell response to persistent Wolbachia infection.Res Microbiol. 2017 Sep;168(7):609-625. doi: 10.1016/j.resmic.2017.04.005. Epub 2017 Apr 21. Res Microbiol. 2017. PMID: 28435138 Free PMC article.
-
Molecular Characterization of LubX: Functional Divergence of the U-Box Fold by Legionella pneumophila.Structure. 2015 Aug 4;23(8):1459-1469. doi: 10.1016/j.str.2015.05.020. Epub 2015 Jul 2. Structure. 2015. PMID: 26146184 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

