Progressive Purkinje cell degeneration in tambaleante mutant mice is a consequence of a missense mutation in HERC1 E3 ubiquitin ligase
- PMID: 20041218
- PMCID: PMC2791161
- DOI: 10.1371/journal.pgen.1000784
Progressive Purkinje cell degeneration in tambaleante mutant mice is a consequence of a missense mutation in HERC1 E3 ubiquitin ligase
Abstract
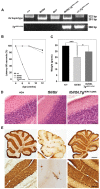
The HERC gene family encodes proteins with two characteristic domains: HECT and RCC1-like. Proteins with HECT domains have been described to function as ubiquitin ligases, and those that contain RCC1-like domains have been reported to function as GTPases regulators. These two activities are essential in a number of important cellular processes such as cell cycle, cell signaling, and membrane trafficking. Mutations affecting these domains have been found associated with retinitis pigmentosa, amyotrophic lateral sclerosis, and cancer. In humans, six HERC genes have been reported which encode two subgroups of HERC proteins: large (HERC1-2) and small (HERC3-6). The giant HERC1 protein was the first to be identified. It has been involved in membrane trafficking and cell proliferation/growth through its interactions with clathrin, M2-pyruvate kinase, and TSC2 proteins. Mutations affecting other members of the HERC family have been found to be associated with sterility and growth retardation. Here, we report the characterization of a recessive mutation named tambaleante, which causes progressive Purkinje cell degeneration leading to severe ataxia with reduced growth and lifespan in homozygous mice aged over two months. We mapped this mutation in mouse chromosome 9 and then performed positional cloning. We found a G<-->A transition at position 1448, causing a Gly to Glu substitution (Gly483Glu) in the highly conserved N-terminal RCC1-like domain of the HERC1 protein. Successful transgenic rescue, with either a mouse BAC containing the normal copy of Herc1 or with the human HERC1 cDNA, validated our findings. Histological and biochemical studies revealed extensive autophagy associated with an increase of the mutant protein level and a decrease of mTOR activity. Our observations concerning this first mutation in the Herc1 gene contribute to the functional annotation of the encoded E3 ubiquitin ligase and underline the crucial and unexpected role of this protein in Purkinje cell physiology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

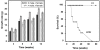




Similar articles
-
Mutation of the HERC 1 Ubiquitin Ligase Impairs Associative Learning in the Lateral Amygdala.Mol Neurobiol. 2018 Feb;55(2):1157-1168. doi: 10.1007/s12035-016-0371-8. Epub 2017 Jan 19. Mol Neurobiol. 2018. PMID: 28102468
-
The HERC proteins and the nervous system.Semin Cell Dev Biol. 2022 Dec;132:5-15. doi: 10.1016/j.semcdb.2021.11.017. Epub 2021 Nov 27. Semin Cell Dev Biol. 2022. PMID: 34848147 Review.
-
Functional and pathological relevance of HERC family proteins: a decade later.Cell Mol Life Sci. 2016 May;73(10):1955-68. doi: 10.1007/s00018-016-2139-8. Epub 2016 Jan 22. Cell Mol Life Sci. 2016. PMID: 26801221 Free PMC article. Review.
-
A new homozygous HERC1 gain-of-function variant in MDFPMR syndrome leads to mTORC1 hyperactivation and reduced autophagy during cell catabolism.Mol Genet Metab. 2020 Sep-Oct;131(1-2):126-134. doi: 10.1016/j.ymgme.2020.08.008. Epub 2020 Sep 4. Mol Genet Metab. 2020. PMID: 32921582
-
HERC1 E3 Ubiquitin Ligase Is Necessary for Autophagy Processes and for the Maintenance and Homeostasis of Vesicles in Motor Nerve Terminals, but Not for Proteasomal Activity.Int J Mol Sci. 2025 Jan 18;26(2):793. doi: 10.3390/ijms26020793. Int J Mol Sci. 2025. PMID: 39859507 Free PMC article.
Cited by
-
Morphology, clearing efficacy, and mTOR dependency of the organelle autophagoproteasome.Eur J Histochem. 2021 Jun 1;65(s1):3220. doi: 10.4081/ejh.2021.3220. Eur J Histochem. 2021. PMID: 34060734 Free PMC article.
-
Mutation of the HERC 1 Ubiquitin Ligase Impairs Associative Learning in the Lateral Amygdala.Mol Neurobiol. 2018 Feb;55(2):1157-1168. doi: 10.1007/s12035-016-0371-8. Epub 2017 Jan 19. Mol Neurobiol. 2018. PMID: 28102468
-
Identification of a quality-control factor that monitors failures during proteasome assembly.Science. 2021 Aug 27;373(6558):998-1004. doi: 10.1126/science.abc6500. Science. 2021. PMID: 34446601 Free PMC article.
-
Whole-genome sequencing analysis in families with recurrent pregnancy loss: A pilot study.PLoS One. 2023 Feb 17;18(2):e0281934. doi: 10.1371/journal.pone.0281934. eCollection 2023. PLoS One. 2023. PMID: 36800380 Free PMC article.
-
Modulation of autoimmune diabetes by N-ethyl-N-nitrosourea- induced mutations in non-obese diabetic mice.Dis Model Mech. 2022 Jun 1;15(6):dmm049484. doi: 10.1242/dmm.049484. Epub 2022 Jun 1. Dis Model Mech. 2022. PMID: 35502705 Free PMC article.
References
-
- Wassef M, Sotelo C, Cholley B, Brehier A, Thomasset M. Cerebellar mutations affecting the postnatal survival of Purkinje cells in the mouse disclose a longitudinal pattern of differentially sensitive cells. Dev Biol. 1987;124:379–389. - PubMed
-
- Bonhomme F, Guénet JL. The laboratory mouse and its wild relatives. In: Lyon MF, Rastan S, Brown SDM, editors. Genetic Variants and Strains of the Laboratory Mouse, 3rd edn. Oxford: Oxford University Press; 1996. pp. 1577–1596.
-
- Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, et al. Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature. 1996;379:736–739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

