Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial
- PMID: 20041484
- PMCID: PMC2819592
- DOI: 10.1002/cncr.24837
Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial
Abstract
Background: The study was conducted to determine whether radiofrequency ablation (RFA) can safely reduce pain from osseous metastatic disease.
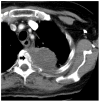
Methods: The single-arm prospective trial included patients with a single painful bone metastasis with unremitting pain with a score >50 on a pain scale of 0-100. Percutaneous computed tomography-guided RFA of the bone metastasis to temperatures >60 degrees C was performed. Endpoints were the toxicity and pain effects of RFA before and at 2 weeks, 1 month, and 3 months after RFA.
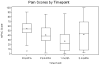
Results: Fifty-five patients completed RFA. Grade 3 toxicities occurred in 3 of 55 (5%) patients. RFA reduced pain at 1 and 3 months for all pain assessment measures. The average increase in pain relief from pre-RFA to 1-month follow-up is 26.3 (95% confidence interval [CI], 17.7-34.9; P < .0001), and the increase from pre-RFA to 3-month follow-up is 16.38 (95% CI, 3.4-29.4; P = .02). The average decrease in pain intensity from pre-RFA to 1-month follow-up was 26.9 (P < .0001) and 14.2 for 3-month follow-up (P = .02). The odds of lower pain severity at 1-month follow-up were 14.0 (95% CI, 2.3-25.7; P < .0001) times higher than at pre-RFA, and the odds at 3-month follow-up were 8.0 (95% CI, 0.9-15.2; P < .001) times higher than at pre-RFA. The average increase in mood from pre-RFA to 1-month follow-up was 19.9 (P < .0001) and 14.9 to 3-month follow-up (P = .005).
Conclusions: This cooperative group trial strongly suggests that RFA can safely palliate pain from bone metastases.
Figures






References
-
- Stoll BA, Parbhoo S, editors. Bone Metastasis: Monitoring and Treatment. New York: Raven Press; 1983.
-
- Twycross RG. Management of pain in skeletal metastases. Clin Ortho. 1995;312:187–196. - PubMed
-
- Janjan NA. Radiation for bone metastases: conventional techniques and the role of systemic pharmaceuticals. Cancer. 1997;80:1628–1645. - PubMed
-
- Quilty PM, Kirk D, Bolger JJ, Dearnaley DP, Lewington VJ, Mason MD, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol. 1994;31:33–40. - PubMed
-
- Serafini AN, Houston SJ, Resche I, Quick DP, Grund FM, Ell PJ, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double blind placebo-controlled trial. J Clin Oncol. 1998;16:1574–1581. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

