Synthesis and characterization of iodinated tetrahydroquinolines targeting the G protein-coupled estrogen receptor GPR30
- PMID: 20041667
- PMCID: PMC2818973
- DOI: 10.1021/jm9011802
Synthesis and characterization of iodinated tetrahydroquinolines targeting the G protein-coupled estrogen receptor GPR30
Abstract
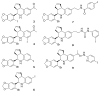
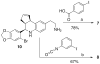
A series of iodo-substituted tetrahydro-3H-cyclopenta[c]quinolines was synthesized as potential targeted imaging agents for the G protein-coupled estrogen receptor GPR30. The affinity and specificity of binding to GPR30 versus the classical estrogen receptors ER alpha/beta and functional responses associated with ligand-binding were determined. Selected iodo-substituted tetrahydro-3H-cyclopenta[c]quinolines exhibited IC(50) values lower than 20 nM in competitive binding studies with GPR30-expressing human endometrial cancer cells. These compounds functioned as antagonists of GPR30 and blocked estrogen-induced PI3K activation and calcium mobilization. The tributylstannyl precursors of selected compounds were radiolabeled with (125)I using the iodogen method. In vivo biodistribution studies in female ovariectomized athymic (NCr) nu/nu mice bearing GPR30-expressing human endometrial tumors revealed GPR30-mediated uptake of the radiotracer ligands in tumor, adrenal, and reproductive organs. Biodistribution and quantitative SPECT/CT studies revealed structurally related differences in the pharmacokinetic profiles, target tissue uptake, and metabolism of the radiolabeled compounds as well as differences in susceptibility to deiodination. The high lipophilicity of the compounds adversely affects the in vivo biodistribution and clearance of these radioligands and suggests that further optimization of this parameter may lead to improved targeting characteristics.
Figures
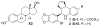

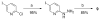
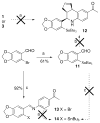
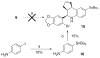
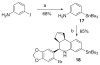
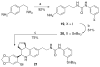
References
-
- Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. - PubMed
-
- Lange CA, Gioeli D, Hammes SR, Marker PC. Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu Rev Physiol. 2007;69:171–199. - PubMed
-
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. - PubMed
-
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

