Ion selectivity of alpha-hemolysin with a beta-cyclodextrin adapter. I. Single ion potential of mean force and diffusion coefficient
- PMID: 20041673
- PMCID: PMC2847479
- DOI: 10.1021/jp906790f
Ion selectivity of alpha-hemolysin with a beta-cyclodextrin adapter. I. Single ion potential of mean force and diffusion coefficient
Abstract
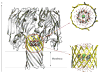
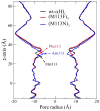
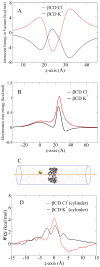
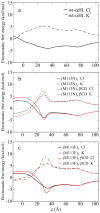
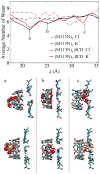
The alpha-hemolysin (alphaHL) is a self-assembling exotoxin that binds to the membrane of a susceptible host cell and causes its death. Experimental studies show that electrically neutral beta-cyclodextrin (betaCD) can insert into the alphaHL channel and significantly increase its anion selectivity. To understand how betaCD can affect ion selectivity, molecular dynamics simulations and potential of mean force (PMF) calculations are carried out for different alphaHL channels with and without the betaCD adapter. A multiscale approach based on the generalized solvent boundary potential is used to reduce the size of the simulated system. The PMF profiles reveal that betaCD has no anion selectivity by itself but can increase the Cl(-) selectivity of the alphaHL channel when lodged into the pore lumen. Analysis shows that betaCD causes a partial desolvation of ions and affects the orientation of nearby charged residues. The ion selectivity appears to result from increased electrostatic interaction between the ion and the channel due to a reduction in dielectric shielding by the solvent. These observations suggest a reasonable explanation of the ion selectivity and provide important information for further ion channel modification.
Figures




References
-
- Laestadius A, Richter-Dahlfors A, Aperia A. Kidney Int. 2002;62:2035–2042. - PubMed
-
- Gentschev I, Dietrich G, Goebel W. Trends in Microbiology. 2002;10:39–45. - PubMed
-
- Rowe SM, Miller S, Sorscher EJ. New England Journal of Medicine. 2005;352:1992–2001. - PubMed
-
- Gouaux E. J Struct Biol. 1998;121:110–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

