Hypoxia/reoxygenation-induced mutations in mammalian cells detected by the flow cytometry mutation assay and characterized by mutant spectrum
- PMID: 20041756
- PMCID: PMC2848454
- DOI: 10.1667/RR1838.1
Hypoxia/reoxygenation-induced mutations in mammalian cells detected by the flow cytometry mutation assay and characterized by mutant spectrum
Abstract
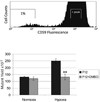
Under hypoxic conditions, cells are more resistant to cell killing by ionizing radiation by a factor of 2.5 to 3, potentially compromising the efficacy of radiotherapy. It has been shown recently that hypoxic conditions alone are sufficient to generate mutations in vitro and in vivo, likely due to the creation of reactive oxygen species (ROS) and a decrease in mismatch and homologous recombination DNA repair activity. These factors are known precursors to the onset of genetic instability and poor prognosis. We have previously characterized the flow cytometry mutation assay and its sensitivity to detect significant mutant fractions induced by genotoxic agents that are not detected by other mammalian assays. Here we measure the mutant fraction induced by hypoxia. CHO A(L) cells cultured at <0.1% O(2) for 24 h generated a significant mutant fraction of 120 x 10(-5) and had growth kinetics and survival characteristics similar to those obtained with other mutagens. We investigated the role of ROS by treating cells with the radical scavenger DMSO, which significantly reduced hypoxia toxicity and mutagenesis. Single cells were sorted from the mutant population, and the resulting clonal populations were stained for five antigens encoded by genes found along chromosome 11 to generate mutant spectra. The mutations were primarily large deletions, similar to those in background mutants, but the frequency was higher. We have demonstrated that hypoxic conditions alone are sufficient to generate mutations in mammalian cells in culture and that the spectrum of mutations is similar to background mutations.
Figures



References
-
- Moulder JE, Rockwell S. Tumor hypoxia: its impact on cancer therapy. Cancer Metastasis Rev. 1987;5:313–341. - PubMed
-
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. - PubMed
-
- Hall EJ. Radiobiology for the Radiologist. 5th. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 91–111.
-
- Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer. 2008;8:180–192. - PubMed
-
- Risom L, Lundby C, Thomsen JJ, Mikkelsen L, Loft S, Friis G, Moller P. Acute hypoxia and reoxygenation-induced DNA oxidation in human mononuclear blood cells. Mutat. Res. 2007;625:125–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

