FXR an emerging therapeutic target for the treatment of atherosclerosis
- PMID: 20041971
- PMCID: PMC3837604
- DOI: 10.1111/j.1582-4934.2009.00997.x
FXR an emerging therapeutic target for the treatment of atherosclerosis
Abstract
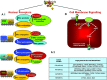
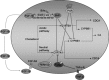
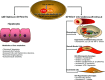
Atherosclerosis is the leading cause of illness and death. Therapeutic strategies aimed at reducing cholesterol plasma levels have shown efficacy in either reducing progression of atherosclerotic plaques and atherosclerosis-related mortality. The farnesoid-X-receptor (FXR) is a member of metabolic nuclear receptors (NRs) superfamily activated by bile acids. In entero-hepatic tissues, FXR functions as a bile acid sensor regulating bile acid synthesis, detoxification and excretion. In the liver FXR induces the expression of an atypical NR, the small heterodimer partner, which subsequently inhibits the activity of hepatocyte nuclear factor 4alpha repressing the transcription of cholesterol 7a-hydroxylase, the critical regulatory gene in bile acid synthesis. In the intestine FXR induces the release of fibroblast growth factor 15 (FGF15) (or FGF19 in human), which activates hepatic FGF receptor 4 (FGFR4) signalling to inhibit bile acid synthesis. In rodents, FXR activation decreases bile acid synthesis and lipogenesis and increases lipoprotein clearance, and regulates glucose homeostasis by reducing liver gluconeogenesis. FXR exerts counter-regulatory effects on macrophages, vascular smooth muscle cells and endothelial cells. FXR deficiency in mice results in a pro-atherogenetic lipoproteins profile and insulin resistance but FXR(-/-) mice fail to develop any detectable plaques on high-fat diet. Synthetic FXR agonists protect against development of aortic plaques formation in murine models characterized by pro-atherogenetic lipoprotein profile and accelerated atherosclerosis, but reduce HDL levels. Because human and mouse lipoprotein metabolism is modulated by different regulatory pathways the potential drawbacks of FXR ligands on HDL and bile acid synthesis need to addressed in relevant clinical settings.
Figures

Similar articles
-
Phosphorylation of hepatic farnesoid X receptor by FGF19 signaling-activated Src maintains cholesterol levels and protects from atherosclerosis.J Biol Chem. 2019 May 31;294(22):8732-8744. doi: 10.1074/jbc.RA119.008360. Epub 2019 Apr 17. J Biol Chem. 2019. PMID: 30996006 Free PMC article.
-
Bile acids: regulation of synthesis.J Lipid Res. 2009 Oct;50(10):1955-66. doi: 10.1194/jlr.R900010-JLR200. Epub 2009 Apr 3. J Lipid Res. 2009. PMID: 19346330 Free PMC article. Review.
-
Bile Acids as Hormones: The FXR-FGF15/19 Pathway.Dig Dis. 2015;33(3):327-31. doi: 10.1159/000371670. Epub 2015 May 27. Dig Dis. 2015. PMID: 26045265 Free PMC article. Review.
-
Protective effects of farnesoid X receptor (FXR) on hepatic lipid accumulation are mediated by hepatic FXR and independent of intestinal FGF15 signal.Liver Int. 2015 Apr;35(4):1133-1144. doi: 10.1111/liv.12456. Epub 2014 Feb 7. Liver Int. 2015. PMID: 25156247 Free PMC article.
-
Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism.Lipids Health Dis. 2018 Dec 19;17(1):286. doi: 10.1186/s12944-018-0939-6. Lipids Health Dis. 2018. PMID: 30567573 Free PMC article.
Cited by
-
Corn silk extract improves cholesterol metabolism in C57BL/6J mouse fed high-fat diets.Nutr Res Pract. 2016 Oct;10(5):501-506. doi: 10.4162/nrp.2016.10.5.501. Epub 2016 Jul 4. Nutr Res Pract. 2016. PMID: 27698957 Free PMC article.
-
Association of serum fibroblast growth factor 19 levels with arteriosclerosis parameters assessed by arterial stiffness and atherogenic index of plasma in patients with type 2 diabetes.Diabetol Metab Syndr. 2020 May 20;12:44. doi: 10.1186/s13098-020-00552-0. eCollection 2020. Diabetol Metab Syndr. 2020. PMID: 32477430 Free PMC article.
-
Bile Acid Metabolism in Liver Pathobiology.Gene Expr. 2018 May 18;18(2):71-87. doi: 10.3727/105221618X15156018385515. Epub 2018 Jan 11. Gene Expr. 2018. PMID: 29325602 Free PMC article. Review.
-
HDL, Atherosclerosis, and Emerging Therapies.Cholesterol. 2013;2013:891403. doi: 10.1155/2013/891403. Epub 2013 May 28. Cholesterol. 2013. PMID: 23781332 Free PMC article.
-
Bile acid nuclear receptor FXR and digestive system diseases.Acta Pharm Sin B. 2015 Mar;5(2):135-44. doi: 10.1016/j.apsb.2015.01.004. Epub 2015 Feb 25. Acta Pharm Sin B. 2015. PMID: 26579439 Free PMC article. Review.
References
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. - PubMed
-
- Makishima M, Lu TT, Xie W, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–16. - PubMed
-
- Kawamata Y, Fujii R, Hosoya M, et al. A G proteincoupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

