Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells
- PMID: 20041972
- PMCID: PMC3822492
- DOI: 10.1111/j.1582-4934.2009.00996.x
Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells
Abstract
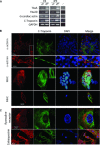
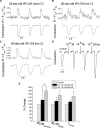
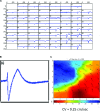
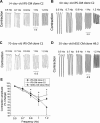
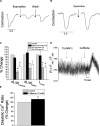
In view of the therapeutic potential of cardiomyocytes derived from induced pluripotent stem (iPS) cells (iPS-derived cardiomyocytes), in the present study we investigated in iPS-derived cardiomyocytes, the functional properties related to [Ca(2+) ](i) handling and contraction, the contribution of the sarcoplasmic reticulum (SR) Ca(2+) release to contraction and the b-adrenergic inotropic responsiveness. The two iPS clones investigated here were generated through infection of human foreskin fibroblasts (HFF) with retroviruses containing the four human genes: OCT4, Sox2, Klf4 and C-Myc. Our major findings showed that iPS-derived cardiomyocytes: (i) express cardiac specific RNA and proteins; (ii) exhibit negative force-frequency relations and mild (compared to adult) post-rest potentiation; (iii) respond to ryanodine and caffeine, albeit less than adult cardiomyocytes, and express the SR-Ca(2+) handling proteins ryanodine receptor and calsequestrin. Hence, this study demonstrates that in our cardiomyocytes clones differentiated from HFF-derived iPS, the functional properties related to excitation-contraction coupling, resemble in part those of adult cardiomyocytes.
© 2011 The Author Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures


References
-
- Wong BW, Rahmani M, Rezai N, et al. Progress in heart transplantation. Cardiovasc Pathol. 2005;14:176–80. - PubMed
-
- Hunt SA, Haddad F. The changing face of heart transplantation. J Am Coll Cardiol. 2008;52:587–98. - PubMed
-
- Habib M, Caspi O, Gepstein L. Human embryonic stem cells for cardiomyogenesis. J Mol Cell Cardiol. 2008;45:462–74. - PubMed
-
- Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. - PubMed
-
- Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

