T2 quantification for improved detection of myocardial edema
- PMID: 20042111
- PMCID: PMC2809052
- DOI: 10.1186/1532-429X-11-56
T2 quantification for improved detection of myocardial edema
Abstract
Background: T2-Weighted (T2W) magnetic resonance imaging (MRI) pulse sequences have been used to detect edema in patients with acute myocardial infarction and differentiate acute from chronic infarction. T2W sequences have suffered from several problems including (i) signal intensity variability caused by phased array coils, (ii) high signal from slow moving ventricular chamber blood that can mimic and mask elevated T2 in sub-endocardial myocardium, (iii) motion artifacts, and (iv) the subjective nature of T2W image interpretation. In this work we demonstrate the advantages of a quantitative T2 mapping technique to accurately and reliably detect regions of edematous myocardial tissue without the limitations of qualitative T2W imaging.
Methods: Methods of T2 mapping were evaluated on phantoms; the best of these protocols was then optimized for in vivo imaging. The optimized protocol was used to study the spatial, view-dependent, and inter-subject variability and motion sensitivity in healthy subjects. Using the insights gained from this, the utility of T2 mapping was demonstrated in a porcine model of acute myocardial infarction (AMI) and in three patients with AMI.
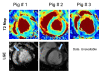
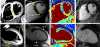
Results: T2-prepared SSFP demonstrated greater accuracy in estimating the T2 of phantoms than multi-echo turbo spin echo. The T2 of human myocardium was found to be 52.18 +/- 3.4 ms (range: 48.96 ms to 55.67 ms), with variability between subjects unrelated to heart rate. Unlike T2W images, T2 maps did not show any signal variation due to the variable sensitivity of phased array coils and were insensitive to cardiac motion. In the three pigs and three patients with AMI, the T2 of the infarcted region was significantly higher than that of remote myocardium.
Conclusion: Quantitative T2 mapping addresses the well-known problems associated with T2W imaging of the heart and offers the potential for increased accuracy in the detection of myocardial edema.
Figures





References
-
- Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF Jr, Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. - DOI - PubMed
-
- Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

