Regulation of Akt(ser473) phosphorylation by choline kinase in breast carcinoma cells
- PMID: 20042122
- PMCID: PMC2806310
- DOI: 10.1186/1476-4598-8-131
Regulation of Akt(ser473) phosphorylation by choline kinase in breast carcinoma cells
Abstract
Background: The serine/threonine kinase PKB/Akt plays essential role in various cellular processes including cell growth and proliferation, metabolism and cell survival. The importance of the Akt pathway is highlighted by the mutation of various components of the pathway such as the PTEN and PI3-kinase (P110alpha) in human cancers. In this paper, we employed an RNA interference library targeting all human kinases to screen for kinases involved in the regulation of Akt activation, in particular serine 473 phosphorylation. Here, we transfected the MDA-MB 468 breast cell line with the human kinome siRNA library and measured Akt activation using an antibody specific for phosphoserine 473 of Akt.
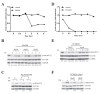
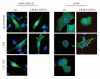
Results: The screen revealed that phosphorylation of Akt(ser473) can be regulated by more than 90 kinases. Interestingly, phosphorylation of Akt(ser473), but not thr308, can be severely reduced by inhibition of Choline kinase activity via siRNA or small molecule inhibitors. We show here that the regulation of Akt phosphorylation by Choline kinase is PI3K-independent. In addition, xenograft tumors treated with Choline kinase inhibitors demonstrated a statistically significant decrease in Akt(ser473) phosphorylation. Importantly, the reduction in phosphorylation correlates with regression of these xenograft tumors in the mouse model.
Conclusion: High Choline kinase expression and activity has previously been implicated in tumor development and metastasis. The mechanism by which Choline kinase is involved in tumor formation is still not fully resolved. From our data, we proposed that Choline kinase plays a key role in regulating Akt(ser473) phosphorylation, thereby promoting cell survival and proliferation.
Figures



Similar articles
-
Inhibition of Akt (ser473) phosphorylation and rapamycin-resistant cell growth by knockdown of mammalian target of rapamycin with small interfering RNA in vascular endothelial growth factor receptor-1-targeting vector.Biol Pharm Bull. 2011;34(5):602-8. doi: 10.1248/bpb.34.602. Biol Pharm Bull. 2011. PMID: 21532145
-
Autocrine signalling through erbB receptors promotes constitutive activation of protein kinase B/Akt in breast cancer cell lines.Breast Cancer Res Treat. 2003 Sep;81(2):117-28. doi: 10.1023/A:1025765215765. Breast Cancer Res Treat. 2003. PMID: 14572154
-
[Effects of Astragalus polysaccharide on proliferation and Akt phosphorylation of the basal-like breast cancer cell line].Zhong Xi Yi Jie He Xue Bao. 2011 Dec;9(12):1339-46. doi: 10.3736/jcim20111210. Zhong Xi Yi Jie He Xue Bao. 2011. PMID: 22152773 Chinese.
-
The potential role of Akt phosphorylation in human cancers.Int J Biol Markers. 2008 Jan-Mar;23(1):1-9. doi: 10.1177/172460080802300101. Int J Biol Markers. 2008. PMID: 18409144 Review.
-
Choline kinases: Enzymatic activity, involvement in cancer and other diseases, inhibitors.Int J Cancer. 2025 Apr 1;156(7):1314-1325. doi: 10.1002/ijc.35286. Epub 2024 Dec 11. Int J Cancer. 2025. PMID: 39660774 Review.
Cited by
-
A-Kinase Anchor Protein 1 deficiency causes mitochondrial dysfunction in mouse model of hyperoxia induced acute lung injury.Front Pharmacol. 2022 Oct 3;13:980723. doi: 10.3389/fphar.2022.980723. eCollection 2022. Front Pharmacol. 2022. PMID: 36263130 Free PMC article.
-
Curcumin enhances cisplatin sensitivity by suppressing NADPH oxidase 5 expression in human epithelial cancer.Oncol Lett. 2019 Aug;18(2):2132-2139. doi: 10.3892/ol.2019.10479. Epub 2019 Jun 14. Oncol Lett. 2019. PMID: 31423287 Free PMC article.
-
Multiple host kinases contribute to Akt activation during Salmonella infection.PLoS One. 2013 Aug 22;8(8):e71015. doi: 10.1371/journal.pone.0071015. eCollection 2013. PLoS One. 2013. PMID: 23990921 Free PMC article.
-
Functional interactions between Choline kinase α, epidermal growth factor receptor and c-Src in breast cancer cell proliferation.Oncogene. 2012 Mar 15;31(11):1431-41. doi: 10.1038/onc.2011.332. Epub 2011 Aug 8. Oncogene. 2012. PMID: 21822308 Free PMC article.
-
Mechanisms for PDGF, a serum cytokine, stimulating loss of corneal keratocyte crystallins.Cornea. 2013 Sep;32(9):1269-75. doi: 10.1097/ICO.0b013e318296e0b9. Cornea. 2013. PMID: 23846408 Free PMC article.
References
-
- Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7(10):776–789. doi: 10.1016/S0960-9822(06)00336-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

