Kinetics and intracellular location of intramolecular disulfide bond formation mediated by the cytoplasmic redox system encoded by vaccinia virus
- PMID: 20042211
- PMCID: PMC2823943
- DOI: 10.1016/j.virol.2009.11.026
Kinetics and intracellular location of intramolecular disulfide bond formation mediated by the cytoplasmic redox system encoded by vaccinia virus
Abstract
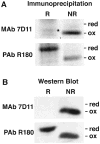
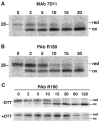
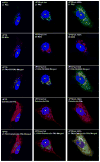
Poxviruses encode a redox system for intramolecular disulfide bond formation in cytoplasmic domains of viral proteins. Our objectives were to determine the kinetics and intracellular location of disulfide bond formation. The vaccinia virus L1 myristoylated membrane protein, used as an example, has three intramolecular disulfide bonds. Reduced and disulfide-bonded forms of L1 were distinguished by electrophoretic mobility and reactivity with monoclonal and polyclonal antibodies. Because disulfide bonds formed during 5 min pulse labeling with radioactive amino acids, a protocol was devised in which dithiothreitol was present at this step. Disulfide bond formation was detected by 2 min after removal of reducing agent and was nearly complete in 10 min. When the penultimate glycine residue was mutated to prevent myristoylation, L1 was mistargeted to the endoplasmic reticulum and disulfide bond formation failed to occur. These data suggested that viral membrane association was required for oxidation of L1, providing specificity for the process.
Published by Elsevier Inc.
Figures



References
-
- Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Pannell LK, Lebowitz J, Fogg C, White C, Moss B, Cohen GH, Eisenberg RJ. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology. 2005;341:59–71. - PubMed
-
- Condit RC, Moussatche N, Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv Virus Res. 2006;66:31–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

