KCNE2 modulation of Kv4.3 current and its potential role in fatal rhythm disorders
- PMID: 20042375
- PMCID: PMC2819024
- DOI: 10.1016/j.hrthm.2009.10.012
KCNE2 modulation of Kv4.3 current and its potential role in fatal rhythm disorders
Abstract
Background: The transient outward current I(to) is of critical importance in regulating myocardial electrical properties during the very early phase of the action potential. The auxiliary beta subunit KCNE2 recently was shown to modulate I(to).
Objective: The purpose of this study was to examine the contributions of KCNE2 and its two published variants (M54T, I57T) to I(to).
Methods: The functional interaction between Kv4.3 (alpha subunit of human I(to)) and wild-type (WT), M54T, and I57T KCNE2, expressed in a heterologous cell line, was studied using patch-clamp techniques.
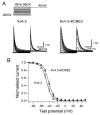
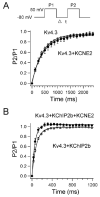
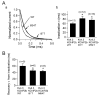
Results: Compared to expression of Kv4.3 alone, co-expression of WT KCNE2 significantly reduced peak current density, slowed the rate of inactivation, and caused a positive shift of voltage dependence of steady-state inactivation curve. These modifications rendered Kv4.3 channels more similar to native cardiac I(to). Both M54T and I57T variants significantly increased I(to) current density and slowed the inactivation rate compared with WT KCNE2. Moreover, both variants accelerated the recovery from inactivation.
Conclusion: The study results suggest that KCNE2 plays a critical role in the normal function of the native I(to) channel complex in human heart and that M54T and I57T variants lead to a gain of function of I(to), which may contribute to generating potential arrhythmogeneity and pathogenesis for inherited fatal rhythm disorders.
Figures
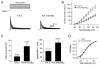

Comment in
-
Cardiac I(to), KCNE2, and Brugada syndrome: promiscuous subunit interactions, or what happens in HEK cells stays in HEK cells?Heart Rhythm. 2010;7(2):206-7. doi: 10.1016/j.hrthm.2009.10.029. Epub 2009 Oct 27. Heart Rhythm. 2010. PMID: 20022816 No abstract available.
References
-
- Kass RS, Freeman LC. Potassium channels in the heart: cellular, molecular, and clinical implications. Trends Cardiovasc Med. 1993;3:149–159. - PubMed
-
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. - PubMed
-
- Abbott GW, Goldstein SA. A superfamily of small potassium channel subunits: form and function of the MinK-related peptides (MiRPs) Q Rev Biophys. 1998;31:357–398. - PubMed
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Sanguinetti MC, Curran ME, Zou AR, et al. Coassembly of KvLQT1 and minK (IKs) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

