Anti-Argonaute RIP-Chip shows that miRNA transfections alter global patterns of mRNA recruitment to microribonucleoprotein complexes
- PMID: 20042474
- PMCID: PMC2811668
- DOI: 10.1261/rna.1905910
Anti-Argonaute RIP-Chip shows that miRNA transfections alter global patterns of mRNA recruitment to microribonucleoprotein complexes
Abstract
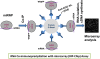
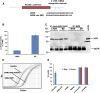
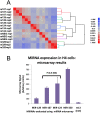
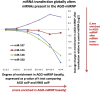
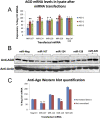
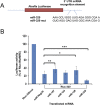
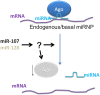
MicroRNAs (miRNAs) play key roles in gene expression regulation by guiding Argonaute (AGO)-containing microribonucleoprotein (miRNP) effector complexes to target polynucleotides. There are still uncertainties about how miRNAs interact with mRNAs. Here we employed a biochemical approach to isolate AGO-containing miRNPs from human H4 tumor cells by co-immunoprecipitation (co-IP) with a previously described anti-AGO antibody. Co-immunoprecipitated (co-IPed) RNAs were subjected to downstream Affymetrix Human Gene 1.0 ST microarray analysis. During rigorous validation, the "RIP-Chip" assay identified target mRNAs specifically associated with AGO complexes. RIP-Chip was performed after transfecting brain-enriched miRNAs (miR-107, miR-124, miR-128, and miR-320) and nonphysiologic control miRNA to identify miRNA targets. As expected, the miRNA transfections altered the mRNA content of the miRNPs. Specific mRNA species recruited to miRNPs after miRNA transfections were moderately in agreement with computational target predictions. In addition to recruiting mRNA targets into miRNPs, miR-107 and to a lesser extent miR-128, but not miR-124 or miR-320, caused apparent exclusion of some mRNAs that are normally associated with miRNPs. MiR-107 and miR-128 transfections also result in decreased AGO mRNA and protein levels. However, AGO mRNAs were not recruited to miRNPs after either miR-107 or miR-128 transfection, confirming that miRNAs may alter gene expression without stable association between particular mRNAs and miRNPs. In summary, RIP-Chip assays constitute an optimized, validated, direct, and high-throughput biochemical assay that provides data about specific miRNA:mRNA interactions, as well as global patterns of regulation by miRNAs.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
