Epstein-Barr virus-encoded Bcl-2 homologue functions as a survival factor in Wp-restricted Burkitt lymphoma cell line P3HR-1
- PMID: 20042495
- PMCID: PMC2826050
- DOI: 10.1128/JVI.01616-09
Epstein-Barr virus-encoded Bcl-2 homologue functions as a survival factor in Wp-restricted Burkitt lymphoma cell line P3HR-1
Abstract
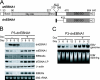
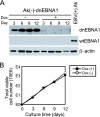
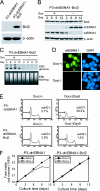
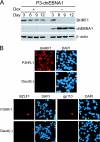
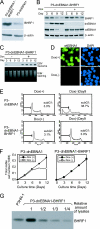
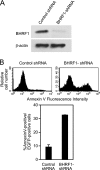
Burkitt lymphoma (BL) is etiologically associated with Epstein-Barr virus (EBV). EBV-positive BL tumors display two latent forms of infection. One is referred to as latency I infection, in which EBV expresses the virus genome maintenance protein EBNA1 as the only viral protein. The other is referred to as Wp-restricted latency and was recently identified in a subset of BL tumors. In these tumors, EBV expresses EBNA1, EBNA3A, EBNA3B, EBNA3C, a truncated form of EBNA-LP, and the viral Bcl-2 homologue BHRF1, all of which are driven by the BamHI W promoter (Wp). To investigate the role of EBV in Wp-restricted BL, we conditionally expressed a dominant-negative EBNA1 (dnEBNA1) mutant which interrupts the virus genome maintenance function of EBNA1 in the P3HR-1 BL cell line. Induction of dnEBNA1 expression caused loss of the EBV genome and resulted in apoptosis of P3HR-1 cells in the absence of exogenous apoptosis inducers, indicating that P3HR-1 cells cannot survive without EBV. Stable transfection of the BHRF1 gene into P3HR-1 cells rescued the cells from the apoptosis induced by dnEBNA1 expression, whereas stable transfection of truncated EBNA-LP, EBNA3A, or EBNA3C did not. Moreover, knockdown of BHRF1 expression in P3HR-1 cells resulted in increased cell death. These results indicate that EBV is essential for the survival of P3HR-1 cells and that BHRF1 functions as a survival factor. Our finding implies a critical contribution of BHRF1 to the pathogenesis of Wp-restricted BLs.
Figures


Similar articles
-
An Epstein-Barr virus anti-apoptotic protein constitutively expressed in transformed cells and implicated in burkitt lymphomagenesis: the Wp/BHRF1 link.PLoS Pathog. 2009 Mar;5(3):e1000341. doi: 10.1371/journal.ppat.1000341. Epub 2009 Mar 13. PLoS Pathog. 2009. PMID: 19283066 Free PMC article.
-
EBNA2-deleted Epstein-Barr virus (EBV) isolate, P3HR1, causes Hodgkin-like lymphomas and diffuse large B cell lymphomas with type II and Wp-restricted latency types in humanized mice.PLoS Pathog. 2020 Jun 15;16(6):e1008590. doi: 10.1371/journal.ppat.1008590. eCollection 2020 Jun. PLoS Pathog. 2020. PMID: 32542010 Free PMC article.
-
Different patterns of Epstein-Barr virus latency in endemic Burkitt lymphoma (BL) lead to distinct variants within the BL-associated gene expression signature.J Virol. 2013 Mar;87(5):2882-94. doi: 10.1128/JVI.03003-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269792 Free PMC article.
-
Potential cellular functions of Epstein-Barr Nuclear Antigen 1 (EBNA1) of Epstein-Barr Virus.Viruses. 2013 Jan 16;5(1):226-40. doi: 10.3390/v5010226. Viruses. 2013. PMID: 23325328 Free PMC article. Review.
-
EBNA2 and Its Coactivator EBNA-LP.Curr Top Microbiol Immunol. 2015;391:35-59. doi: 10.1007/978-3-319-22834-1_2. Curr Top Microbiol Immunol. 2015. PMID: 26428371 Review.
Cited by
-
Epstein-Barr virus: more than 50 years old and still providing surprises.Nat Rev Cancer. 2016 Dec;16(12):789-802. doi: 10.1038/nrc.2016.92. Epub 2016 Sep 30. Nat Rev Cancer. 2016. PMID: 27687982 Review.
-
Epstein-Barr virus (EBV) provides survival factors to EBV+ diffuse large B-cell lymphoma (DLBCL) lines and modulates cytokine induced specific chemotaxis in EBV+ DLBCL.Immunology. 2017 Dec;152(4):562-573. doi: 10.1111/imm.12792. Epub 2017 Aug 11. Immunology. 2017. PMID: 28699226 Free PMC article.
-
Role of Epstein-Barr Virus C Promoter Deletion in Diffuse Large B Cell Lymphoma.Cancers (Basel). 2021 Feb 1;13(3):561. doi: 10.3390/cancers13030561. Cancers (Basel). 2021. PMID: 33535665 Free PMC article.
-
A computationally designed inhibitor of an Epstein-Barr viral Bcl-2 protein induces apoptosis in infected cells.Cell. 2014 Jun 19;157(7):1644-1656. doi: 10.1016/j.cell.2014.04.034. Cell. 2014. PMID: 24949974 Free PMC article.
-
Epstein-Barr Virus Encoded BCL2, BHRF1, Downregulates Autophagy by Noncanonical Binding of BECN1.Biochemistry. 2023 Oct 17;62(20):2934-2951. doi: 10.1021/acs.biochem.3c00225. Epub 2023 Sep 30. Biochemistry. 2023. PMID: 37776275 Free PMC article.
References
-
- Anderton, E., J. Yee, P. Smith, T. Crook, R. E. White, and M. J. Allday. 2008. Two Epstein-Barr virus (EBV) oncoproteins cooperate to repress expression of the proapoptotic tumour-suppressor Bim: clues to the pathogenesis of Burkitt's lymphoma. Oncogene 27:421-433. - PubMed
-
- Askew, D. S., R. A. Ashmun, B. C. Simmons, and J. L. Cleveland. 1991. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene 6:1915-1922. - PubMed
-
- Austin, P. J., E. Flemington, C. N. Yandava, J. L. Strominger, and S. H. Speck. 1988. Complex transcription of the Epstein-Barr virus BamHI fragment H rightward open reading frame 1 (BHRF1) in latently and lytically infected B lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 85:3678-3682. - PMC - PubMed
-
- Bissonnette, R. P., F. Echeverri, A. Mahboubi, and D. R. Green. 1992. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature 359:552-554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

