GLTSCR2/PICT-1, a putative tumor suppressor gene product, induces the nucleolar targeting of the Kaposi's sarcoma-associated herpesvirus KS-Bcl-2 protein
- PMID: 20042497
- PMCID: PMC2826064
- DOI: 10.1128/JVI.00757-09
GLTSCR2/PICT-1, a putative tumor suppressor gene product, induces the nucleolar targeting of the Kaposi's sarcoma-associated herpesvirus KS-Bcl-2 protein
Abstract
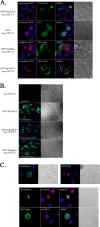
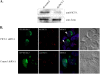
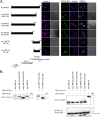
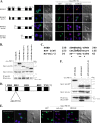
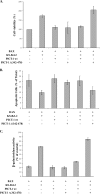
KS-Bcl-2, encoded by Kaposi's sarcoma-associated herpesvirus (KSHV), is a structural and functional homologue of the Bcl-2 family of apoptosis regulators. Like several other Bcl-2 family members, KS-Bcl-2 protects cells from apoptosis and autophagy. Using a yeast two-hybrid screen and coimmunoprecipitation assays, we identified a novel KS-Bcl-2-interacting protein, referred to as protein interacting with carboxyl terminus 1 (PICT-1), encoded by a candidate tumor suppressor gene, GLTSCR2. Confocal laser scanning microscopy revealed nucleolar localization of PICT-1, whereas KS-Bcl-2 was located mostly at the mitochondrial membranes with a small fraction in the nucleoli. Ectopic expression of PICT-1 resulted in a large increase in the nucleolar fraction of KS-Bcl-2, and only a minor fraction remained in the cytoplasm. Furthermore, knockdown of endogenous PICT-1 abolished the nucleolar localization of KS-Bcl-2. However, ectopically expressed PICT-1 did not alter the cellular distribution of human Bcl-2. Subsequent analysis mapped the crucial amino acid sequences of both KS-Bcl-2 and PICT-1 required for their interaction and for KS-Bcl-2 targeting to the nucleolus. Functional studies suggest a correlation between nucleolar targeting of KS-Bcl-2 by PICT-1 and reduction of the antiapoptotic activity of KS-Bcl-2. Thus, these studies demonstrate a cellular mechanism to sequester KS-Bcl-2 from the mitochondria and to downregulate its virally encoded antiapoptotic activity. Additional characterization of the interaction of KS-Bcl-2 and PICT-1 is likely to shed light on the functions of both proteins.
Figures
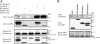

Similar articles
-
Nucleolar localization of GLTSCR2/PICT-1 is mediated by multiple unique nucleolar localization sequences.PLoS One. 2012;7(1):e30825. doi: 10.1371/journal.pone.0030825. Epub 2012 Jan 23. PLoS One. 2012. PMID: 22292050 Free PMC article.
-
The Viral Bcl-2 Homologs of Kaposi's Sarcoma-Associated Herpesvirus and Rhesus Rhadinovirus Share an Essential Role for Viral Replication.J Virol. 2017 Feb 28;91(6):e01875-16. doi: 10.1128/JVI.01875-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053098 Free PMC article.
-
K15 protein of Kaposi's sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function.J Virol. 2002 Jan;76(2):802-16. doi: 10.1128/jvi.76.2.802-816.2002. J Virol. 2002. PMID: 11752170 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus and Kaposi's sarcoma.Microbes Infect. 2000 May;2(6):671-80. doi: 10.1016/s1286-4579(00)00358-0. Microbes Infect. 2000. PMID: 10884618 Review.
-
In search of a function for BCLAF1.ScientificWorldJournal. 2010 Jul 20;10:1450-61. doi: 10.1100/tsw.2010.132. ScientificWorldJournal. 2010. PMID: 20661537 Free PMC article. Review.
Cited by
-
Autophagy and immunity - insights from human herpesviruses.Front Immunol. 2012 Jul 4;3:170. doi: 10.3389/fimmu.2012.00170. eCollection 2012. Front Immunol. 2012. PMID: 22783253 Free PMC article.
-
Nucleolar stress induces ubiquitination-independent proteasomal degradation of PICT1 protein.J Biol Chem. 2014 Jul 25;289(30):20802-12. doi: 10.1074/jbc.M114.571893. J Biol Chem. 2014. PMID: 24923447 Free PMC article.
-
Modification of Nuclear Compartments and the 3D Genome in the Course of a Viral Infection.Acta Naturae. 2020 Oct-Dec;12(4):34-46. doi: 10.32607/actanaturae.11041. Acta Naturae. 2020. PMID: 33456976 Free PMC article.
-
Imaging Hallmarks of Sarcoma Progression Via X-ray Computed Tomography: Beholding the Flower of Evil.Cancers (Basel). 2022 Oct 19;14(20):5112. doi: 10.3390/cancers14205112. Cancers (Basel). 2022. PMID: 36291896 Free PMC article. Review.
-
Mitochondrial Proteins Coded by Human Tumor Viruses.Front Microbiol. 2018 Feb 6;9:81. doi: 10.3389/fmicb.2018.00081. eCollection 2018. Front Microbiol. 2018. PMID: 29467726 Free PMC article. Review.
References
-
- Andersen, J. S., Y. W. Lam, A. K. Leung, S. E. Ong, C. E. Lyon, A. I. Lamond, and M. Mann. 2005. Nucleolar proteome dynamics. Nature 433:77-83. - PubMed
-
- Barboule, N., I. Truchet, and A. Valette. 2005. Localization of phosphorylated forms of Bcl-2 in mitosis: co-localization with Ki-67 and nucleolin in nuclear structures and on mitotic chromosomes. Cell Cycle 4:590-596. - PubMed
-
- Boisvert, F. M., S. van Koningsbruggen, J. Navascues, and A. I. Lamond. 2007. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 8:574-585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

